Industry Dynamic
On June 14, The State Council Information Office held a briefing to introduce the key tasks of deepening the medical and health system reform in 2024. Huang Guo, deputy director of the State Food and Drug Admin...
[View details]
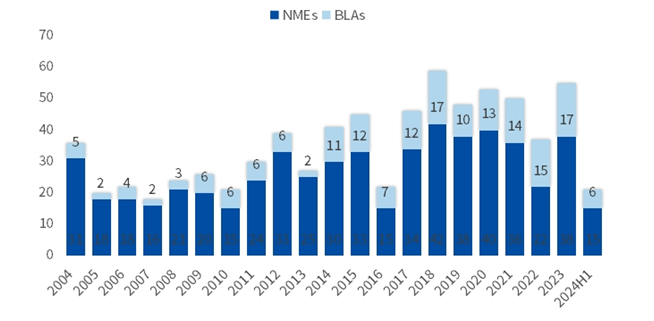
In the first half of 2024, 21 new Class 1 drugs were approved by the Food and Drug Administration (FDA) and 26 new Class 1 drugs were approved by Chinese National Medical Products Administration (NMPA). This ar...
[View details]
After 10 years of painstaking research, the original preclinical small molecule new drugs jointly developed by the research team of East China Normal University and Fudan University in the field of anti-tumor c...
[View details]
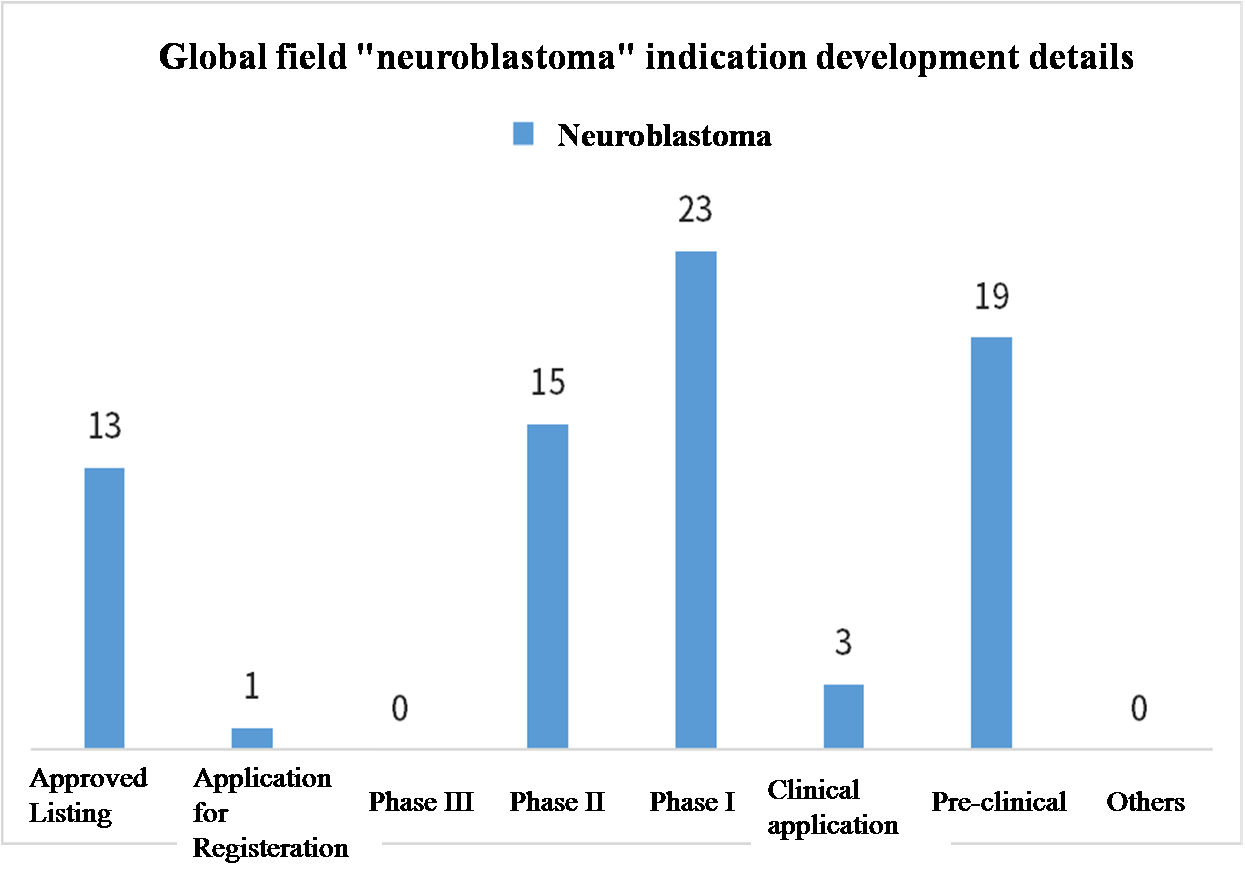
In terms of details, the release of the "second batch of rare disease catalog" involves a total of 86 rare diseases, superimposed under the first batch of 121 rare diseases, two batches of catalogs have include...
[View details]







