Guided by natural objectivity and respect for the laws of life, we approach diseases from the perspectives of holistic and functional medicine, exploring effective molecules within natural substances.
Natural substances (animals, plants, microorganisms, and minerals) have always accompanied human evolution, and human genetic memory allows for the habitual acceptance of effective components in natural substances. Exploring active substances from non-toxic plants to activate the body's immune system and metabolic regulation mechanism for disease treatment is a promising approach and represents the core R&D philosophy of the Jiuzhang Biotech team.
Natural medicines, derived directly from non-toxic plants with their original structures as active ingredients, even without modification, are inherently drug-like and harmless to the human body, such as vitamins.
Drugs based on the original structures of natural plants, which have been chemically synthesized and modified through chemical means (such as removing toxic functional groups, and modifying or adding pharmacologically active functional groups), have improved clinical efficacy and decreased toxicity and side effects. These drugs remain irreplaceable and are continuously being discovered for new indications, such as aspirin and metformin.

It is broadly effective against glioma, lung squamous cell carcinoma, esophageal squamous cell carcinoma, cervical squamous cell carcinoma, oral squamous cell carcinoma, colorectal cancer, and other types of cancer.
Based on the research of mechanism, it can be used for the treatment of multiple types of diseases, such as autoimmune diseases and metabolic disorders.
Patients who live a normal life for over 10 years include:
Patients of tongue squamous cell carcinoma, lung squamous cell carcinoma, and glioblastoma with postoperative recurrence, etc.;
Patients who still live a normal life after early interventional treatment include: patients with small-cell lung cancer, cervical squamous cell carcinoma, esophageal squamous cell carcinoma, etc.
For more details, please refer to the “Diseases and Treatments” section.
Clinical data indicates excellent safety, with no other drug-related adverse reactions besides muscle induration at the injection site.
It demonstrates synergistic effects and toxicity attenuation when combined with multiple chemotherapeutic agents, targeted therapies, and immunotherapeutic agents.
Mechanism studies have shown that CHA is more effective in the early treatment of tumors.
Improving quality of life for the patients with increased KPS values
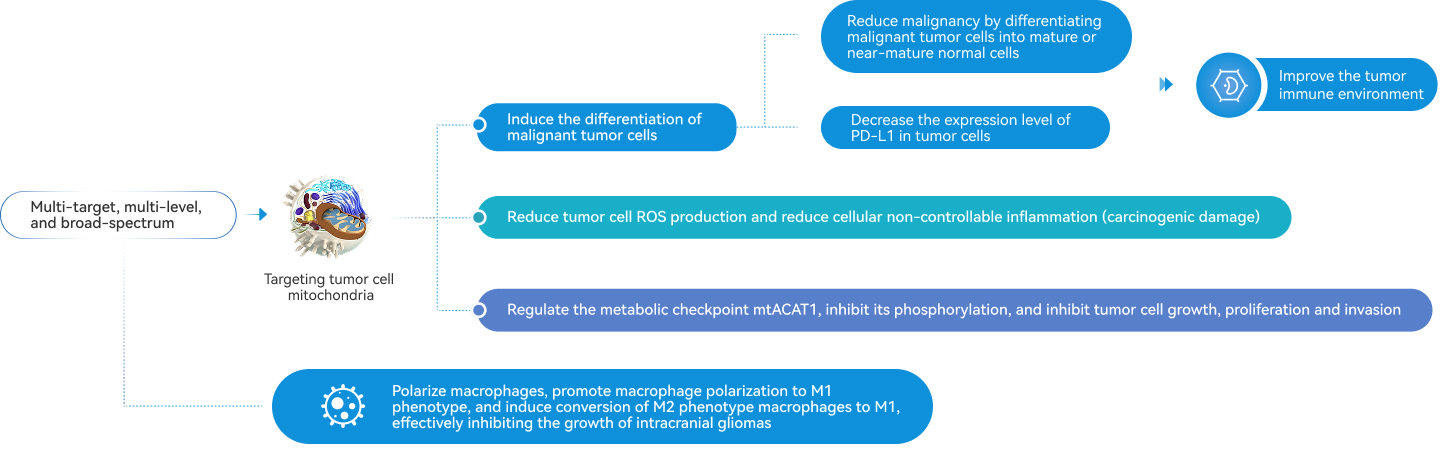
Exerting anti-tumor effects by multi-target, multi-level
Inhibiting mtACAT1 phosphorylation
Releasing immuno-suppression to stimulate immunity
Inducing tumor cell differentiation
disorders
biological
functions
diseases
effects
diseases
An Asia-Pacific branch of MHG, located in Bangkok and Koh Samui coastline in Thailand, is equipped with Germany's top medical testing and treatment equipment. It is led by Germany's top physicians and primarily serves for the high-net-worth individuals in Europe and America. MHG, specializing in natural therapy, functional medicine and integrative medicine, provides optimum customized cancer prevention and treatment schemes which are suitable for people who have needs such as catering to those seeking medical aesthetics, longevity, holistic health, and tumor prevention and treatment.