Glioblastoma has been included in the second batch of rare diseases list in China
In September 2023, the National Health Commission and other six departments jointly formulated the "Second Batch of Rare Diseases Catalogue", which is not only the long-awaited good news for a large number of families with rare diseases in China, but also the hope of countless families with rare diseases. On the other hand, the release of the catalogue also provides a sufficient legal basis for China to strengthen the management of rare diseases, improve the diagnosis and treatment level of rare diseases, and safeguard the health rights and interests of rare patients, and has important guiding significance for the promotion of the diagnosis and treatment of rare diseases in the future, promoting the accessibility of drugs, and improving the patient protection system.
In terms of details, the release of the "second batch of rare disease catalog" involves a total of 86 rare diseases, superimposed under the first batch of 121 rare diseases, two batches of catalogs have included 207 rare diseases, of which glioblastoma has been included in the second batch of rare disease catalog in China.
At the same time, in the second batch of rare disease catalog, there are also a lot of key content worth mentioning, that is, more than 29 rare diseases of 40+ innovative drugs have been approved in the domestic market, 10+ new drugs for rare diseases are about to be listed, and more than 18 rare diseases in the field of domestic pharmaceutical company layout.
Driven by the huge market of 300 million rare disease patients in the world, although new drugs for rare diseases have been successfully launched at home and abroad, there are still more than 90% of rare diseases without treatment, which has also led to "new drug research for rare diseases" becoming one of the key areas of medical exploration and therapy research and development at this stage. In fact, the relevant data also shows that in recent years, more than 50% of the new drugs approved by the FDA's Center for Drug Evaluation and Research (CDER) are rare diseases, and the study of rare disease indications accounts for 30% of the global clinical pipeline of new drugs, which shows its major trend.
In contrast, the domestic approved new drugs for rare diseases and their huge clinical demand are still in an imbalance, but there is still a long way to go. However, the good news is that the relevant agencies attach great importance to the above issues, and more and more new drugs for rare diseases will gradually be launched in the future, which is worth looking forward to.
For the details of approved new drugs for rare diseases, taking neuroblastoma as an example, according to drug Intelligence data - Global drug Analysis System and public information show that there are more than 70 new drugs involved in the indication worldwide, as of October 2023, preliminary statistics have 13 drugs overseas approved for listing, 15 drugs in phase II clinical, 23 drugs in phase I clinical. There are also 22 new drugs in the preclinical and clinical application stages.
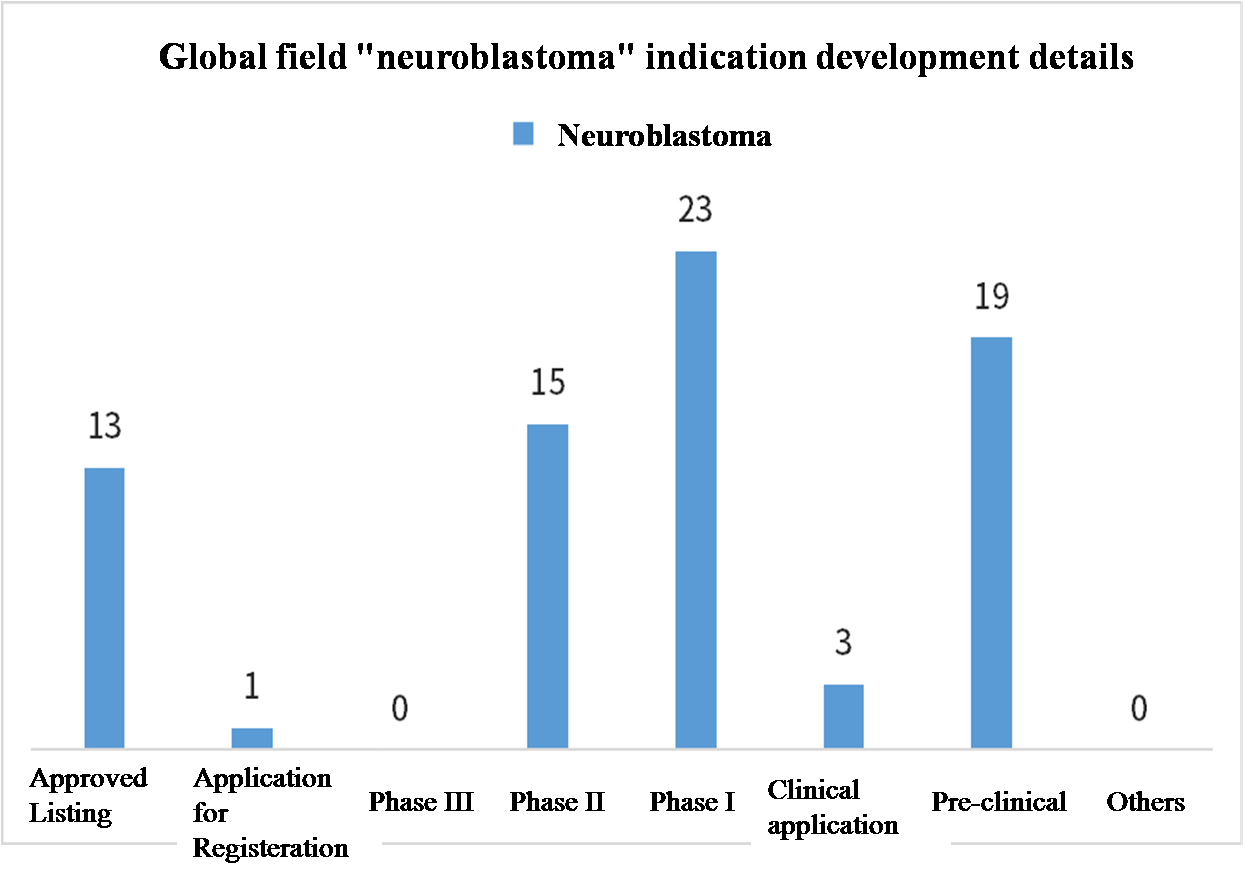
Data source: Yaozhi Data, open data collation
For the field of rare diseases, the satisfaction of any clinical need is perhaps more precious and worth celebrating than in other disease areas. Of course, the listing of new drugs is not the end point of treatment for rare diseases, and under the help of the second batch of rare disease catalogs, the approved new drugs for rare diseases are more likely to be endorsed by core policies such as medical insurance, further strengthening the accessibility and cost optimization of drugs for rare patients.
Under the specific analysis of the second batch of rare disease catalogs, it can be seen that the proportion of new drugs for rare diseases in the development of new drugs for cancer is relatively large, which is also the first time in this catalog. According to statistics, the proportion of tumor field in the first batch of rare disease catalogs is only about 3%, while this catalog has directly increased to 28%, and more than 20 rare disease tumors have been included.
In this case, some innovative cancer drugs inadvertently cover some rare disease indications, which is also one of the reasons for the emergence of a large number of new rare disease drugs in the catalog. In this regard, the industry expressed a certain degree of concern, after all, for the field of cancer, both its treatment and payment guarantee mechanism seem to have been relatively perfect, and the lack of treatment for more hereditary rare diseases should be the most considered content of the rare disease catalog. In addition, the policy overlap of tumor + rare disease and speculation under the rapid approval of rare disease indications may also become new problems in the future.
However, no matter what the situation is, as far as the current catalog of rare diseases as a whole is concerned, the combination model of rare diseases + tumors will always be an inimitable obstacle in the future, and it may be more promising to promote industrial progress under the attempt, and at least as far as the layout of relevant innovative drugs in the catalog of rare diseases is concerned, whether it is the accessibility of drugs for patients, or the enthusiasm of more drug companies in the future. Its driving effect is undoubtedly positive.







