The following article is from Bo Yao, Author: Forest
In the first half of 2024, 21 new Class 1 drugs were approved by the Food and Drug Administration (FDA) and 26 new Class 1 drugs were approved by Chinese National Medical Products Administration (NMPA). This article will take stock of these new drugs approved in China and the United States respectively, and compare the similarities and differences in drug types, indications and other aspects of new drugs approved in the two countries.
By the end of June 2024, the Food and Drug Administration's (FDA) Center for Drug Evaluation Research (CDER) had approved a total of 21 new drugs, including 17 chemical drugs and six biologic drugs, which is slightly lower than the same period in 2023, but still above the historical average.
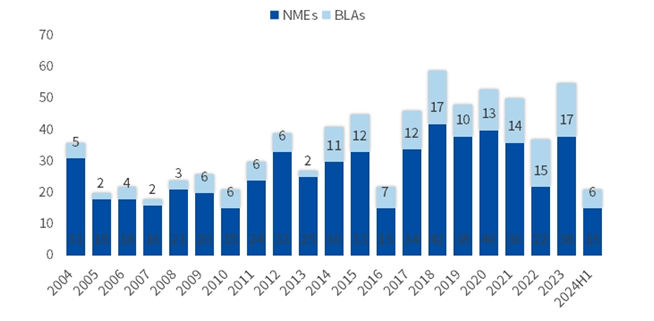
Figure 1. Number of new drugs approved by the FDA over the years
Table 1. New drugs approved by FDA CDER in 2024H1
Name | Active Ingredients | Indication | Enterprise | Date of Approval |
Micromolecule | ||||
ZELSUVMI | BERDAZIMER SODIUM | Molluscum Contagiosum | LNHC | 1/5/24 |
EXBLIFEP | CEFEPIME;ENMETAZOBACTAM | Complex urinary tract infection; Pyelonephritis | ALLECRA THERAPEUTICS SAS | 2/22/24 |
REZDIFFRA | RESMETIROM | MASH | MADRIGAL | 3/14/24 |
TRYVIO | APROCITENTAN | Hypertension | IDORSIA PHARMA LTD | 3/19/24 |
DUVYZAT | GIVINOSTAT | Duchenne Muscular Dystrophy | ITALFARMACO SA | 3/21/24 |
VAFSEO | VADADUSTAT | Anemia from Chronic Kidney Disease | AKEBIA THERAPEUTICS INC | 2024-03-27 |
VOYDEYA | DANICOPAN | Paroxysmal Nocturnal Hemoglobinuria(PNH) | AstraZeneca | 3/29/24 |
ZEVTERA | CEFTOBIPROLE MEDOCARIL SODIUM | Multiple Infection | BASILEA PHARM ALLSCH | 4/3/24 |
OJEMDA | TOVORAFENIB | Low Grade Glioma in Children | DAY ONE BIOPHARMACEUTICALS | 4/23/24 |
XOLREMDI | MAVORIXAFOR | WHIM Syndrome | X4 PHARMACEUTICALS | 4/26/24 |
IQIRVO | ELAFIBRANOR | Primary Biliary Cholangitis(PBC) | IPSEN BIOPHARM LTD | 6/10/24 |
SOFDRA | SOFPIRONIUM | Primary Axillary Hyperhidrosis | BOTANIX SB INC. | 6/18/24 |
OHTUVAYRE | ENSIFENTRINE | Chronic Obstructive Pulmonary Disease(COPD) | Verona Pharma | 6/26/24 |
Imaging Agent | ||||
LUMISIGHT | PEGULICIANINE | Adjuvant Detection During Breast Cancer Surgery | LUMICELL | 4/17/24 |
Oligonucleotides | ||||
RYTELO | IMETELSTAT SODIUM | Myelodysplastic Syndrome(MDS) | GERON | 6/6/24 |
Monoclonal Antibody | ||||
TEVIMBRA | TISLELIZUMAB | Esophageal Squamous Cancer | BeiGene | 3/13/24 |
PIASKY | CROVALIMAB-AKKZ | Paroxysmal Nocturnal Hemoglobinuria(PNH) | Roche | 6/20/24 |
Other Biological Drugs | ||||
WINREVAIR | SOTATERCEPT-CSRK | Pulmonary Arterial Hypertension | Merck | 3/26/24 |
ANKTIVA | Nogapendekin alfa inbakicept-pmln | Non-muscular Invasive Bladder Cancer | ALTOR BIOSCIENCE | 4/22/24 |
IMDELLTRA | TARLATAMAB-DLLE | Non-small Cell Lung Cancer | Amgen | 5/16/24 |
LETYBO | LETIBOTULINUMTOXINA-WLBG | Moderate to Severe Interglabellar Lines in Adults | HUGEL INC | 2/29/24 |
Note: The list includes many new drugs and some biologics approved by CDER, excluding vaccines, allergenic products, blood and blood products, plasma derivatives, cell and gene therapy products, and other products approved by the Center for Biologics Evaluation and Research in 2024.
By the end of June 2024, the Center for Drug Evaluation and Research (CDE) of the Chinese National Medical Products Administration (NMPA) had approved a total of 26 new drugs, including 15 chemical drugs, 8 biological drugs and 3 traditional Chinese medicines, an increase over the same period in 2023 (24).
Table 2. Class 1 new drugs approved by 2024H1 NMPA
Name | Enterprise | Indication | Date of Approval |
Micromolecule | |||
Janagliflozin Tablets | Huisheng biology | Type 2 Diabetes | 1/16/24 |
Tygelidine Fumarate Injection | Hengrui Medicine | Moderate to Severe Pain after Abdominal Surgery | 1/30/24 |
Tollatinib Capsules | Kezhou | Melanoma | 3/12/24 |
Ipkepam Hydrochloride Capsules | Novartis AG NVS | Adult Paroxysmal Sleep Sex is Disease of Haemoglobin(PNH) | 4/24/24 |
Entinostat Tablets | Yi Teng Jing Ang | HR-positive, HER-2-negative, Locally Advanced or Metastatic Breast Cancer that Relapsed or Progressed after Endocrine Therapy | 4/24/24 |
Annicotinib Fumarate Capsules | CHIATAI Tianqing | Ros1-positive, locally advanced or metastatic non-small-cell lung cancer | 4/24/24 |
Ripresinib Capsules | BMS | Ros1-positive, locally advanced or metastatic non-small-cell lung cancer | 5/8/24 |
Benzene Sulfonic Acid in Cleveland in | Haisco | Diabetic Peripheral Neuropathic Pain | 5/15/24 |
Rezivertinib Capsules | Beta Pharma | Always the EGFR - TKI treatment or disease progression after treatment, and that there are confirmed by the detection of EGFR T790M mutation positive locally advanced or metastatic non-small-cell lung cancer (NSCLC) | 5/15/24 |
Ivonac Citrate Capsules | CHIATAI Tianqing | Alk-positive, Locally Advanced or Metastatic Non-small-cell Lung Cancer | 6/11/24 |
Oritinib Mesylate Tablets | SANHOME | EGFR T790M mutation-positive locally advanced or metastatic non-small cell lung cancer | 6/11/24 |
Golidocitinib Capsules | Dizal Pharma | Recurrent or Refractory Peripheral T-cell Lymphoma | 6/18/24 |
Cogletin Tablets | Haisco | Type 2 Diabetes | 6/18/24 |
Sulbactam Sodium for Injection/Dolobactam Sodium for Injection Package | Entasis Therapeutics/Zai Lab | Hospital-acquired bacterial pneumonia caused by susceptible isolates of the baumann-Acinetobacter calcoaceticus complex in patients 18 years of age or older(HABP)、Ventilator-associated bacterial pneumonia(VABP) | 5/15/24 |
Polypeptide | |||
Betabatide Citrate Injection | Bio-Thera | Acute Coronary Syndrome | 6/25/24 |
Echo insulin Injection | Novo Nordisk | Type 2 Diabetes | 6/18/24 |
Monoclonal Antibody | |||
Rencanizumab Injection | Eisai/Bojian | Alzheimer Disease | 1/5/24 |
Covarizumab Injection | Roche | Paroxysmal Nocturnal Hemoglobinuria | 2/6/24 |
Bemosubezumab Injection | CHIATAI Tianqing | Extensive-stage small-cell Lung Cancer | 4/30/24 |
Zemelovir Mazzorizumab Injection | Xingmeng Biology | Passive Immunotherapy for Persons Exposed to Rabies Virus | 6/4/24 |
Enlongabizumab Injection | CSPC Pharmaceutical Group | Platinum-based chemotherapy failure of PD - L1 expression positive recurrence or metastasis of cervical cancer | 6/25/24 |
Double-resistant | |||
Ivor Xidan anti-injection | Akesobio | Combinated pemetrexed and carboplatin for after treatment with EGFR - TKI progress of EGFR mutation positive locally advanced or metastatic non squamous non-small cell lung cancer | 5/21/24 |
CAR-T | |||
Zewaukee Orenseid Injection | Carsgen Therapeutics | Recurrent or Refractory Multiple Myeloma | 2/23/24 |
Traditional Chinese Medicine | |||
Clear Pills on Catechu | Qi Jin Pharmaceutical Industry | Dental Ulcer | 1/8/24 |
Jiuwei Antitussive Oral Liquid | BRIMED | Acute trachea - bronchitis of TCM syndrome differentiation is a wind heat cough | 2/20/24 |
Qinwei Granule | Chengdu Hwaseo Natural Medicine | Acute Gouty Arthritis | 3/12/24 |
Similarities and differences between China and the United States indications, contrast analysis on the types of drugs
In terms of the number of approvals, NMPA approved 26 new drugs in the first half of this year, nearly 20% more than the 21 new drugs approved by CDER in the United States. If out of traditional Chinese medicine with Chinese characteristics and does not belong to the category of CDER approval of cell therapy, it is 22 NMPA approval number of drug, one more than American CDER approval of new drugs. In terms of drug types, small molecules were the main type of approved new drugs in both China and the United States, accounting for more than half of the total. The second is the development of more mature antibody drugs. In addition, new drugs such as specific T cells double adapter, double resistance, CAR - T therapy have dabbled in China and the United States, but a small number.
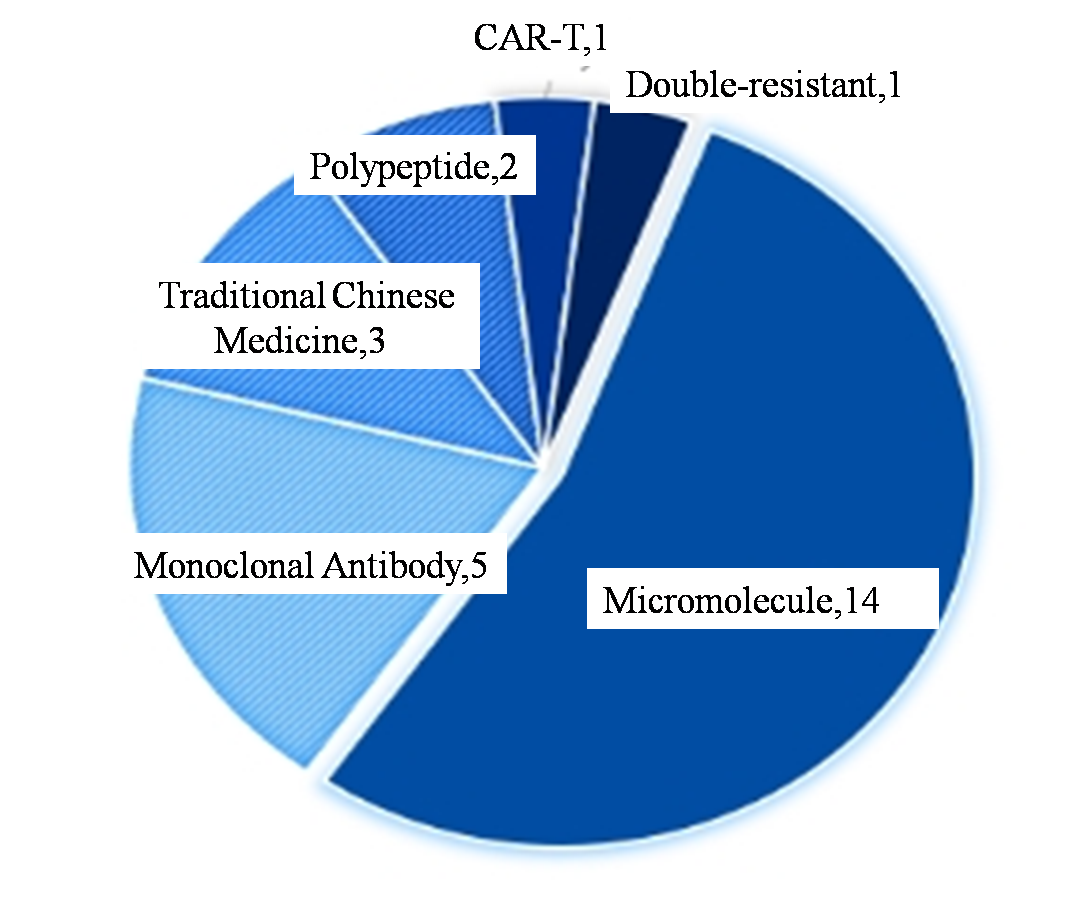
Figure 2. Drug type distribution of New Drugs approved by CDER in the first Half of 2024
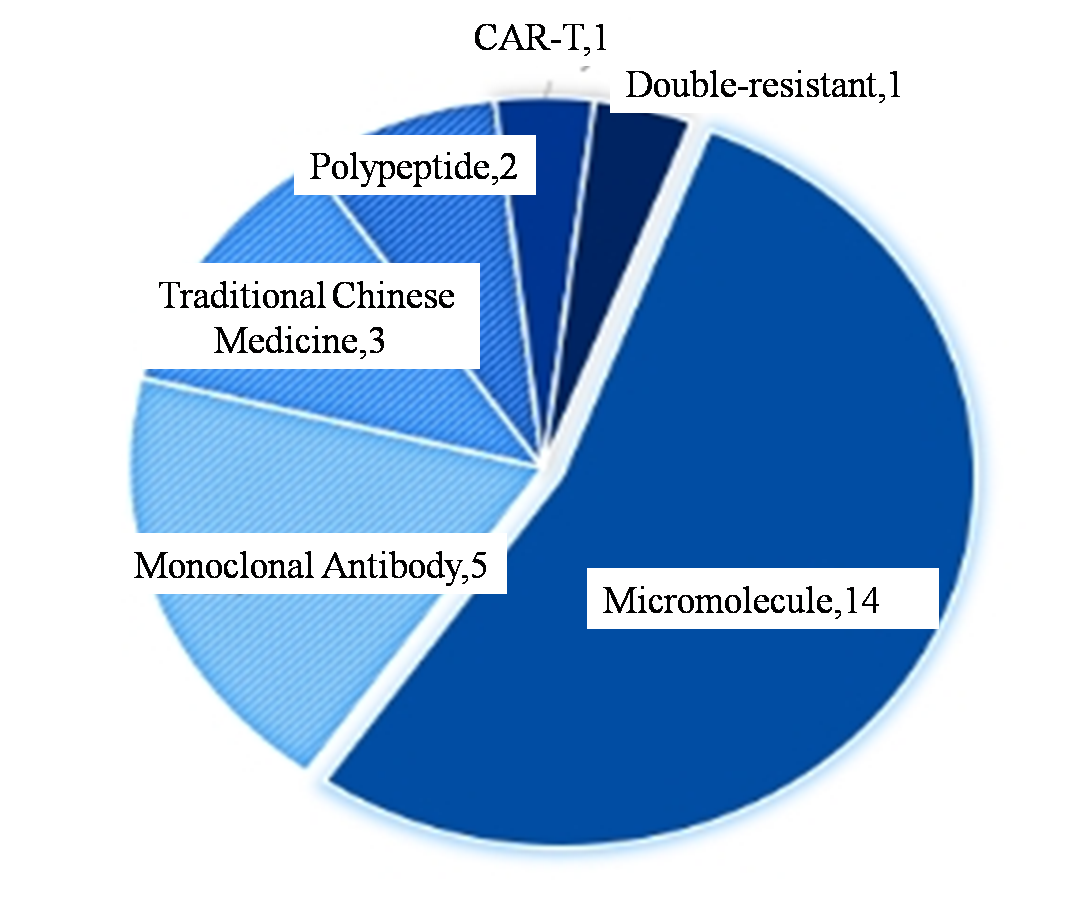
Figure 3. In the first half of 2024 NMPA approval of new drugs drug distribution type
To some extent, there is a certain time lag between the innovative technology in the field of drug research and the drug technology approved. At present, the more popular ADC, CAR-T and other fields have not been reflected in the approval level of new drugs. From the perspective of the current trend of new drug research and development from small molecule drugs to biological drugs, the number of subsequent biological drugs approved may be higher and higher. From the point of indications, the approval of new drugs are antineoplastic drugs in most, but the proportion is higher, close to 50%, and 29% in the United States. From the perspective of specific cancer, China approved seven of the 12 tumor drugs for lung cancer (6 non-small cell lung cancer, 1 small cell lung cancer) treatment, blood tumor 2, cervical cancer, breast cancer, melanoma each one. However, the cancer types of approved new drugs in the United States are scattered, including esophageal cancer, breast cancer, glioma and so on. This a series of differences of China and the United States, it is perhaps one of the reasons for domestic drug homogeneity, hug can be popular, but emphasize differentiation development, such as abbvie generally to establish core disease area advantages, for domestic innovation medicine enterprise may be more worth learning. Second, anti-infection, blood disease, metabolic disease, cardiovascular disease, medication, it is also the hot area of new drug research and development. In addition, rare diseases as the new drug development and approval of key areas, approval of new drugs in the first half of 2024 has 10 new drugs won a orphan drug certification, including four rare tumor drug, two rare blood disease medication, and four other rare diseases and medical treatment. Approved in China, the first half of 2024 in class 1 new drug to market, only 2 import 1 kind of rare disease drugs approved to treat adult patients with paroxysmal sleep sex disease of hemoglobinuria (PNH), there have been no domestic independent research and development of rare diseases of new drugs. Of course, some new drugs for rare diseases have been approved for import under category 5.1 new drugs, which are not included in the statistical scope of this article. To some extent, this may also mean that China's new drug "source innovation" will be insufficient. For rare diseases and other areas with urgent clinical demand, but because of market space, technical difficulties and other issues, the progress is slow, which may need to be strengthened.
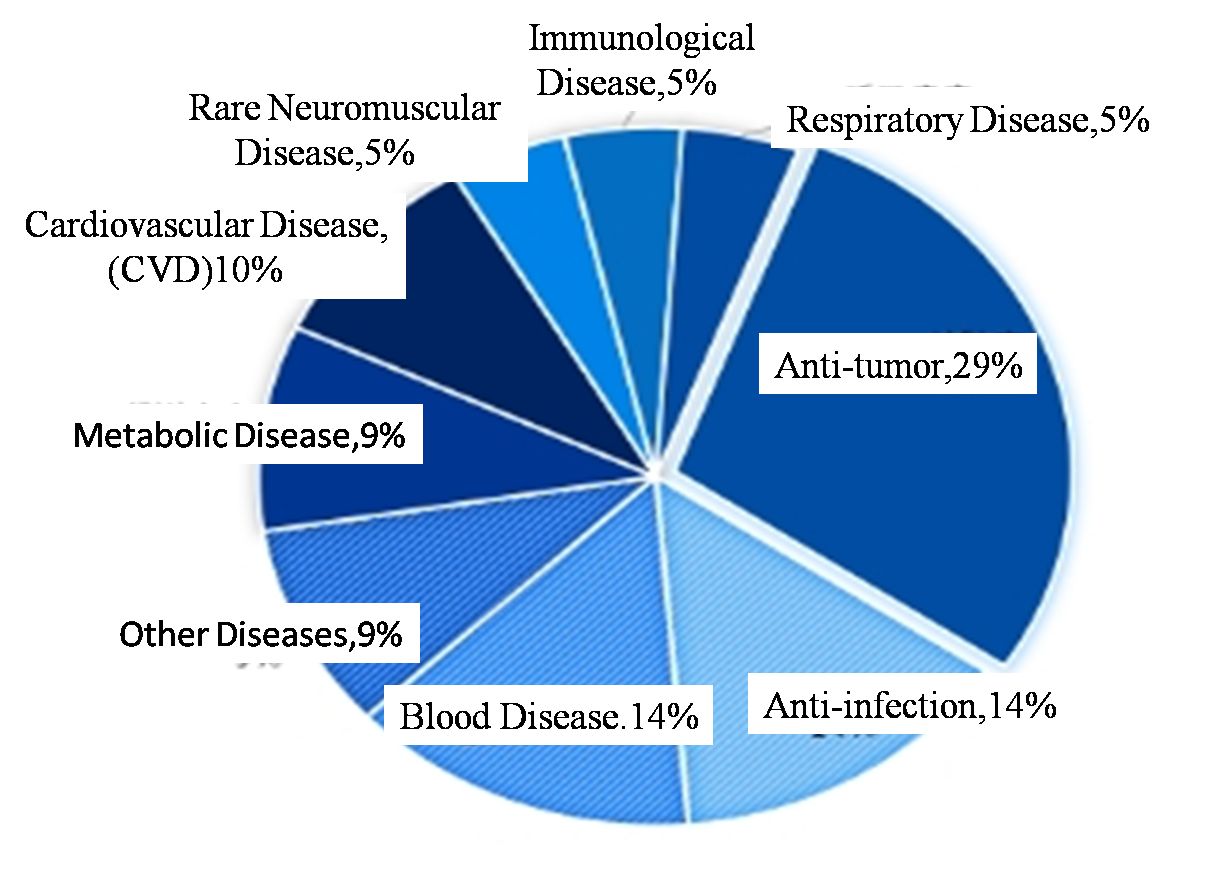
Figure 4. Disease Field Distribution of New Drugs approved by CDER in the first Half of 2024
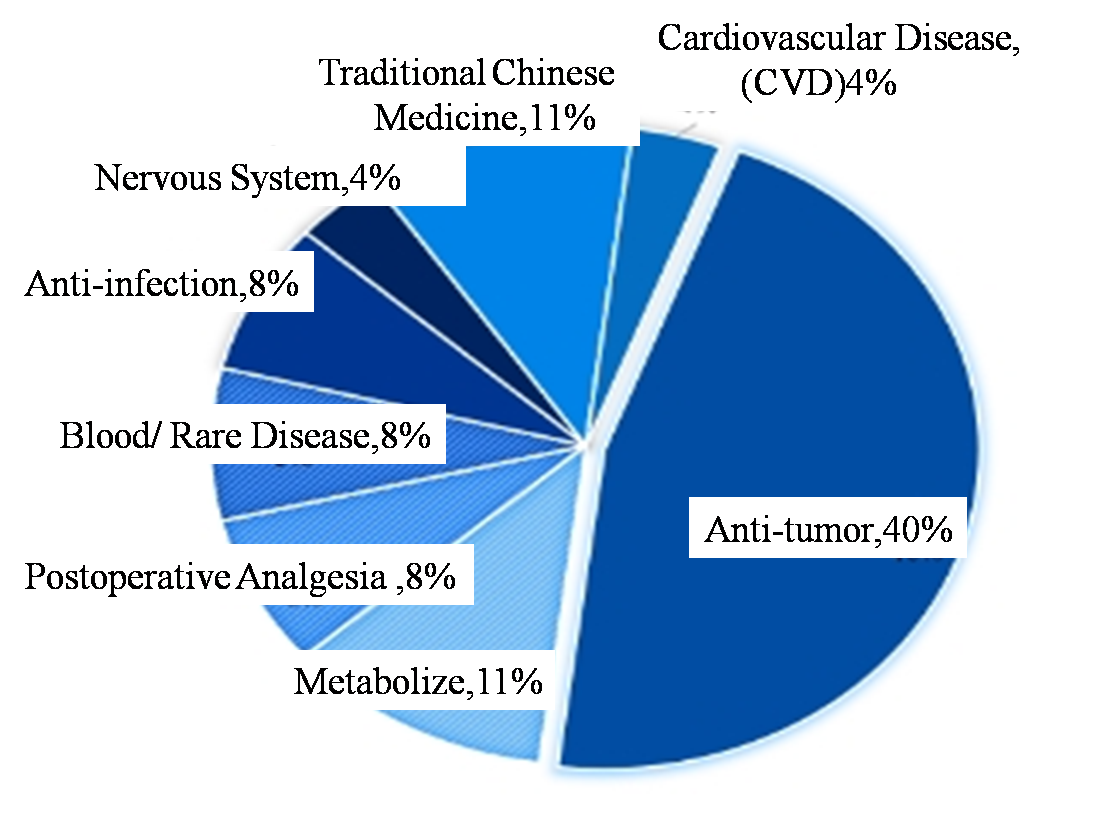
Figure 5. Disease Field Distribution of NMPA approved New Drugs in the First Half of 2024
From the company point of view, Piasky (crovalimab, bevacizumab), a new generation of C5 circulating antibody developed by Roche, has been approved for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) in China and the United States. The FDA approval of new drugs a few from multinational drug companies, such as Merck, astrazeneca, amgen, most biotech companies from the United States, like MADRIGAL, AKEBIA, ALTOR BIOSCIENCE, etc. NMPA approval of new drugs is also a small number of from multinational drug companies, such as BMS, novartis, most Chinese pharmaceutical companies and biotechnology companies, such as hengrui pharmaceutical, nolato, group, zhengda, times of pharmaceutical industry, but the weather is fine and kanfen biological pharmaceutical, etc. In terms of review methods, 10 new drugs were approved in the United States through the fast-track approval system in the first half of 2024, accounting for 48% of all approved new drugs. China has nine new drugs through prior approval has been approved, accounted for 35% of all new drugs approved, visible rapid examination and approval, prior approval is the most important ways of new drug approval.
Summary
In recent years, the number of new drugs approved by NMPA has increased rapidly under the accelerated marketing and registration procedures such as special approval, breakthrough treatment, conditional approval, and priority approval. In 2022, the NMPA approved 23 class 1 new drugs, and this number increased to 40 in 2023. In the first half of this year, 26 class 1 new drugs have been approved, exceeding the number of class 1 new drugs approved by the US FDA, and the number of new drugs approved for the whole year is expected to hit a new high. However, in terms of indication, the domestic new drugs are mainly concentrated in the field of anti-tumor, especially lung cancer. However, rare diseases and rare tumors account for a large proportion of new drugs approved by FDA. The differences with the domestic and international market environment, the innovation medicine development length. For the present, foreign markets more encouraged to explore some new targets and indications, and to compare the new targets, drug firms also tend to put out to sea in search of opportunity. In the future, it is believed that with the policy support and the improvement of the payment environment, China is expected to usher in more innovative therapies.







