“2017 China BioMed Innovation and Investment Conference” was held in SuZhou Jinji Lake International Conference Center On October 29, 2017. Sichuan Jiuzhang Biotech Co, Ltd (hereafter referred to as Jiuzhang) released Phase I clinical trial data on the safety, tolerability and pharmacokinetics of chlorogenic acid for injection in the treatment of advanced malignant glioma.
In 1897, two British scientists Osbome and Campebell found that sunflowers contained a compound that could cause the sunflower protein to turn black; Corter et al named the compound Chlorogenic acid in 1909; Rud-kin and Nelson confirmes the structure of Chlorogenic acid in 1947; in 2000, Jiuzhang began researching Chlorogenic acid as a therapeutic drug for treatment of major diseases (cancer), and obtained clinical approval documents issued by CFDA (Chemical medicine class 1).
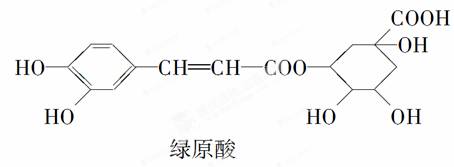
The molecular weight of Chlorogenic acid is 354.31. Chlorogenic acid is a phenolic acid which is composed of caffeic acid and quinic acid, it is a kind of phenylpropanoid compound produced by aerobic respiration of plants (shikimic acid pathway), which is rich in honeysuckle and Eucommiaceae plants.
Depending on the production of high purity chlorogenic acid in kilograms, Jiuzhang identified and isolated the sensitized plant protein, quantified 0.1% impurity in chlorogenic acid, and completed the transformation of chlorogenic acid from extract to API. Jiuzhang has confirmed in academic and medical fields that chlorogenic acid is not a source of sensitization and that it is an extremely valuable natural medicine.
"Chlorogenic acid for injection" is a kind of natural small molecule immunotherapeutic agent, which has a strong pharmacological activity, low toxicity, and clear treatment mechanism.
22 invention patents and 1 utility model patents were obtained by Jiuzhang focusing on the chlorogenic acid production process, structural transformation, indications and other fields.
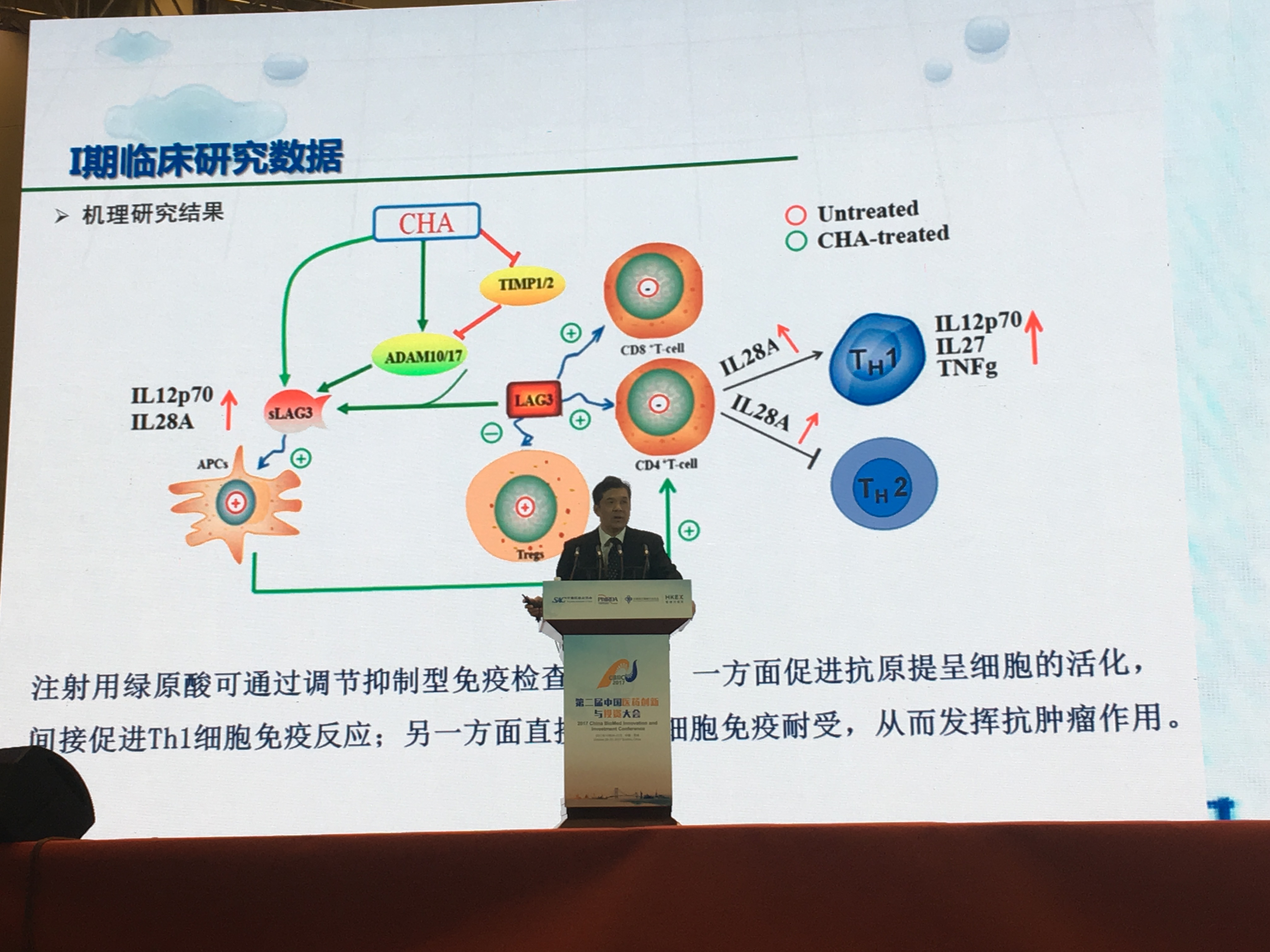
In order to study the pharmacokinetics and molecular mechanism of chlorogenic acid, Jiuzhang and the Chinese Academy of Medical Sciences Institute of Drug Research has established a platform for chlorogenic acid research and development. Professor XiaoguangChen (Well-known as efficacy and pharmacology experts, Ph.D.)and her team research the anti-tumor mechanism of chlorogenic acid at multi-levels (In January 2017, an article titled “Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype” was published at Scientific Reports detailing and demonstrating the mechanism by which chlorogenic acid for injection promotes malignant cell apoptosis and inhibits tumor growth). The latest study reveals that chlorogenic acid show potential on both promoting APC activity and reversing T cell immune tolerance by regulating the immunological checkpoint LAG-3, which may contribute to improve the tumor immune microenvironment and play anti-tumor effect.
The "Phase I Clinical Trials of Safety, Tolerance and Pharmacokinetic Studies of injection of chlorogenic acid treatment Malignant Brain Gliomas for Injection", hosted by Prof. Li Wenbin (PI) at Beijing Millennium Monument Hospitalhas completed. Chlorogenic acid for injection has been shown to be safe and well tolerated in patients with high-grade malignant glioma who have been relapsed after surgery and chemoradiotherapy failure (disease progression). More than 50% subjects after using chlorogenic acid for injection obtained a benefit. Some of themgot a target lesion shrink or disappear, the treatment efficiency is higher than the clinical program design expectations. Jiuzhang is now preparing for the Phase II clinical studies and striving for the conditional approval.
Considering the chlorogenic acid anti-tumor-mechanism study result and its anti-tumor characteristics, a Ib / IIa clinical trial program has been moved into the implementation phase focusing on the small cell lung cancer (SCLC), Squamous non-small cell lung cancer (NSCLC) and adeniform NSCLC in order to start a better development on the broad-spectrum value of chlorogenic acid under the leadership of inventor Jie Zhang.







