The Second China BioMed Innovation and Investment Conference was held at the Suzhou Jinjihu International Conference Center on October 29, 2017, on which the Jiuzhang Biotech released the data of phase I clinical trial study on the safety, tolerance and pharmacokinetics of chlorogenic acid for injection in treating advanced malignant brain glioma.
In 1897, two British scientists Osbome and Campbell discovered a compound in sunflowers that can blacken sunflower seeds proteins; Corter et el named this compound as chlorogenic acid in 1909; Rud-kin and Nelson identified the chemical structure of chlorogenic acid in 1947; after 103 years, in 2000, the study team of Sichuan Jiuzhang Biotech Co., Ltd. (hereinafter referred to as Jiuzhang Biotech) for the first time systematically investigated the chlorogenic acid monomer as a drug for treating major diseases (malignancies) and had obtained clinical approval (Class I chemical drugs) from the CFDA (CHINA FOOD AND DRUG ADMINISTRATION) in 2013.
Chlorogenic acid (molecular weight: 354.31) is a depside composed of caffeic acid and quinic acid as well as a phenylpropanoid produced during aerobic respiration of plants (via shikimic acid). It is abundant in the plants of Caprifoliaceae and Eucommiaceae.
Based on the kilogram-scale production of high purity chlorogenic acid, Jiuzhang Biotech identified and isolated allergenic vegetable proteins from the chlorogenic acid, qualitatively and quantitatively measured the 0.1% impurities in the chlorogenic acid, successfully transformed the chlorogenic acid extract to chlorogenic acid monomer and academically and medically defined the chlorogenic acid as an extremely valuable natural drug instead of a sensitizing source.
Chlorogenic acid for injection is a natural small molecule immunotherapeutic agent with strong pharmacological activity, low toxic and side effect, clear target and clear mechanism of action.
Jiuzhang Biotech has obtained 24 granted invention patents (as of December 31,2017) and 1 utility model patent in the fields of manufacturing process, structural modification and disease treatment.
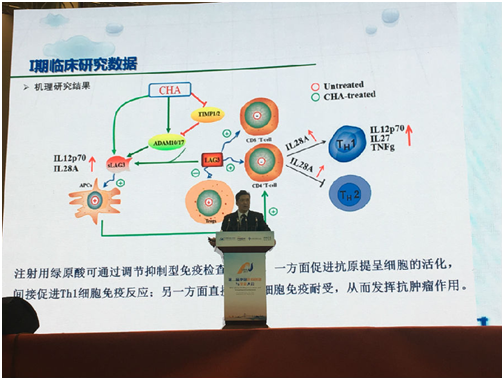
On the pharmacodynamics and molecular mechanism of chlorogenic acid, Jiuzhang Biotech and the Institute of Materia Medica affiliated to Chinese Academy of Medical Science has built a strategic partnership and jointly established a high-end level platform for chlorogenic acid research and development. Prof. Chen, Xiaoguang (a well-known pharmacodynamic and pharmacological expert in China) and his team further investigated the mechanism of anti-tumor action from various aspects (in January 2017, they published an article in Scientific Reports titled Chlorogenic Acid Inhibits Glioblastoma Growth Through Transforming Macrophage from M2 to M1 Type by repolarization, which elaborated and demonstrated the mechanism of chlorogenic acid for injection to promote the apoptosis of malignant tumor cells and inhibit tumor growth). The results of recent mechanism of action research showed that by regulating the immune checkpoint LAG-3, chlorogenic acid not only promoted the activity of antigen-presenting cells, but also reverse the T-cell immune tolerance, thus improving the tumor immune microenvironment and exerting the anti-tumor effect, it is a broad-spectrum drug with very low toxicity.
In Beijing Shijitan Hospital, the Phase I Clinical Trial Study on the Safety, Tolerability and Pharmacokinetics of Chlorogenic Acid for Injection in Treating Malignant Brain Glioma with Prof. Li Wenbin as the Principal Investigator (PI) has been completed. The enrolled subjects were patients with high-grade malignant brain gliomas refractory to international standard of care including surgeries and chemoradiotherapy (progressed disease), among whom the chlorogenic acid for injection showed good safety and tolerance. More than 50% patients with such end-stage highly malignant tumors may benefit from the chlorogenic acid for injection; some subjects may have target lesions in brain tissue reduced or disappeared, showing an efficacy beyond the expectations designed in the protocol. At present, the phase II clinical study has been initiated, expecting early commercialization and availability to the majority of cancer patients.
Based on the results of the anti-tumor mechanism studies and to better exploit the broad-spectrum antitumor value of chlorogenic acid for injection, with the broad-spectrum anti-tumor characteristics of chlorogenic acid, Jiuzhang Team, under the leadership of the inventor and chairman Zhang, Jie, developed the stage Ib/IIa clinical trial protocol of lung cancer for advanced patients with small cell lung cancer, lung squamous carcinoma and lung adenocarcinoma who relapse after surgery and were refractory to conventional treatments. This protocol is currently ready to to implemented.







