Sichuan Jiuzhang Biotech Co., Ltd. has employed the chlorogenic acid monomer for treatment of major diseases (cancers) for the first time in the world, and has secured the clinical approval documents for chlorogenic acid for injection in August 2013 (approval numbers: 2013L01855 and 2013L01856). The current clinical study progress is as follows:
1. Completed - An Exploratory Study to Evaluate the Safety in Human and Dominant Tumor Spectrum as well as Treatment Trends of Chlorogenic Acid for Injection for Treatment of Advanced Solid Tumors
As of July 2016, Jiuzhang Biotech had completed an exploratory study to evaluate the safety in human and dominant tumor spectrum of chlorogenic acid for injection in Beijing Cancer Hospital. Subjects included in this clinical study were all patients with advanced malignant solid tumors who had been refractory to conventional chemoradiotherapy and whose disease had progressed, for whom there was few efficacious treatment drug option in the clinic. In this trial, safety data of chlorogenic acid for injection used in human was collected, and the efficacy trend of chlorogenic acid for injection for treatment of lung, liver, prostate, colorectal and gastric cancers was studied. Results showed that chlorogenic acid for injection was safe and well tolerated in clinical application without obvious toxicity and side effects; the enrolled subjects who had received chlorogenic acid for injection were evaluated and statistically analyzed for efficacy, and during this trial, 75% of subjects had stable disease (SD), 83.3% of subjects continued having stable disease (SD) according to post-withdrawal efficacy evaluation, and 75% of subjects had steadily improved quality of life (as evaluated by EQ5D scale and FACT scale), and 50% of subjects had significantly relieved symptom of pain. Results showed that, as evaluated by RECIST1.0 criteria, the clinical benefit response was 50.0%, which exceeded the investigator's expectation. In summary, chlorogenic acid for injection had demonstrated a safe, effective and broad spectrum of clinical use.
2. Completed - A Phase I Clinical Study on Chlorogenic Acid for Injection for Treatment of Malignant Gliomas
According to the results of tumor spectrum obtained from the exploratory clinical study conducted in the Beijing Cancer Hospital, combined with the mechanistic study of chlorogenic acid for injection for the treatment of malignancies conducted by the Institute of Materia Medica, China Academy of Medical Sciences, Jiuzhang Biotech had determined the dominant tumor species for chlorogenic acid application, and had carried out a phase I clinical trial on chlorogenic acid for injection for treatment of advanced malignant gliomas in Beijing Shijitan Hospital, Capital Medical University to collect the safety data, limit of tolerance and pharmacokinetic parameters of chlorogenic acid for injection in the clinical application and investigate the therapeutic effect of chlorogenic acid for injection on gliomas, thereby providing a basis for clinical studies for the phase II clinical trial application for conditional marketing authorization.
The phase I clinical trial to investigate the safety, tolerability and pharmacokinetics of chlorogenic acid for injection for treatment of malignant gliomas had been completed on September 15, 2017.
Data from the phase I clinical trial showed that monotherapy with chlorogenic acid for injection was safe (with minimal toxicity and side effects and the only adverse reaction of muscle induration caused by long-term injection), and well tolerated for its long-term use (continuous administration was tolerated in the majority of subjects who experienced induration) in patients with advanced, recurrent and high-grade malignant gliomas. All participating subjects were patients with recurrent high-grade (grade III - IV) malignant brain tumors who had been refractory to international standard treatment such as surgery and chemoradiotherapy (disease progression); in the effective dose group, the median overall survival for subjects with grade IV gliomas was 21.4 months, while the internationally recognized median overall survival for subjects at this stage was only 3 - 4.6 months (data source: NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancer). It was exciting that the outcomes of treatment with chlorogenic acid for injection had far exceeded the international standard.
During the latest follow-up (February 26, 2018), it was found that subjects who received continuous administration had a complete response (CR, disappearance of all lesions) or a partial response (PR, lesion shrinkage by more than 50%) upon treatment evaluation, which was exciting. Currently, patients with recurrent high-grade gliomas who had failed in standard treatment were in an irreversible state of “SD-PD-death”(that is, patients would progress from stable disease (SD) to disease progression (PD) to death, which currently is irreversible with any drug) with almost no CR or PR worldwide; in clinical practice, it was also found that the combination therapy regimen of chemotherapy agents such as procarbazine, lomustine and vincristine (PCV regimen) could cause tumor shrinkage in a small number of patients even in the early stage, but didn’t prolong the overall survival. In this trial, the subjects had a complete response or partial response, were in good living condition, and had significantly prolonged survival. This efficacy response was far beyond the investigator's expectation, and was a significant breakthrough!
3. Ongoing - A Phase II/III Clinical Study on Chlorogenic Acid for Injection for Treatment of Malignant Gliomas
Study Name | Director of Clinical Study | Leader Unit | Primary Study Endpoint | Study Status |
A Randomized, Controlled, Open-label, Multi-center Phase II/III Clinical Study to Evaluate the Efficacy and Safety of Chlorogenic Acid for Injection for Treatment of Grade IV Glioblastoma (GBM) | Prof. Li Wenbin | Beijing Tiantan Hospital, Capital Medical University | To assess overall survival (OS) in patients with grade IV glioblastoma treated with chlorogenic acid for injection
| Ongoing |
(1) Total Design:Randomized, Controlled, Open-label, Multi-center
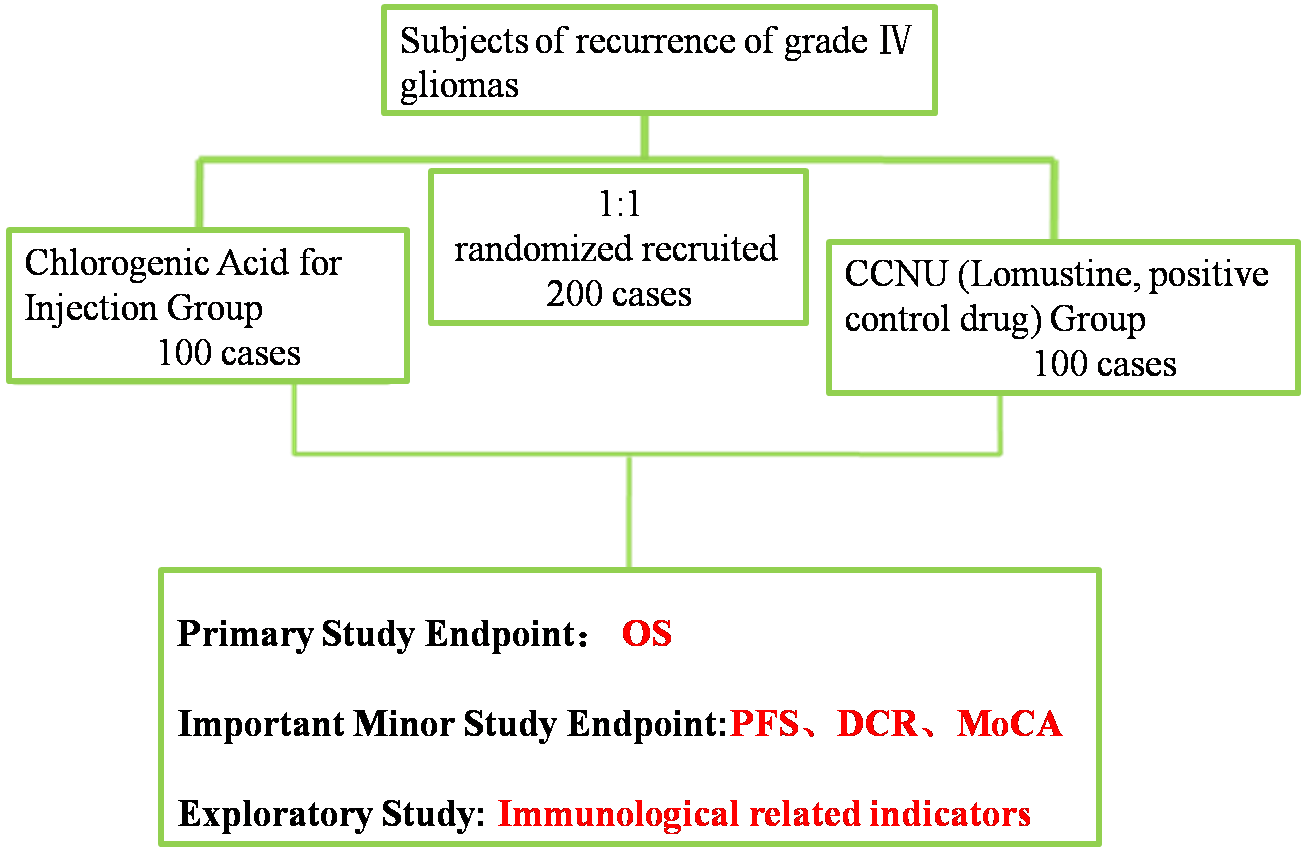
(2) Member units of Phase II/III Clinical Study to Evaluate the Efficacy and Safety of Chlorogenic Acid for Injection for Treatment of Grade IV Glioblastoma (GBM)
NO. | Center Name | Major Researcher | Department | Address |
01 | Beijing Tiantan Hospital, Capital Medical University | Li Wenbin | Neural Tumor Comprehensive Ward | Beijing |
02 | Peking Union Medical College Hospital | Ma Wenbin | Neurosurgery Department | Beijing |
03 | Jiangsu Province Hospital | You Yongping | Neurosurgery Department | Nanjing |
04 | Harbin Medical University Cancer Hospital | Su Jun | Neurosurgery Department | Harbin |
05 | Affiliated Hospital of Ningxia Medical University | Xia Hechun | Neurosurgery Center Department | Yinchuan |
06 | Chongqing Cancer Hospital | Ca Run | Cerebral Surgery Department | Chongqing |
07 | Second People’s Hospital of Shenzhen | Li Weiping | Neurosurgery Department | Shenzhen |
08 | Tianjin Huanhu Hospital | Jiang Wei | Radiotherapy Department | Tianjin |
09 | Sichuan Provincial People's Hospital | Zeng Ming | Oncology Department | Chengdu |
10 | Union Hospital Affiliated to Huazhong University of Science and Technology | Yang Kunyu | Head and Neck Oncology Department | Wuhan |
11 | Shanxi Provincial People's Hospital | Ji Hongming | Neurosurgery Department | Taiyuan |
4. Planned - A Phase Ib/Ⅱa Clinical Study on Chlorogenic Acid for Injection for Treatment of Squamous Cell Lung Cancer and Small Cell Lung Cancer
Lung cancer is a cancer with the highest morbidity and mortality in solid tumors, and includes small cell lung cancer (accounting for 15% of all lung cancer cases) and non-small cell lung cancer, i.e. lung adenocarcinoma and squamous cell lung cancer (accounting for 85% of all lung cancer cases). Currently, various drugs for treatment of lung cancers mainly target lung adenocarcinoma in non-small cell lung cancers (accounting for 50% of non-small cell lung cancer cases), while small cell lung cancer and squamous cell lung cancer, which similarly account for a large proportion in lung cancers, lack therapeutic drugs. According to the exploration of preliminary clinical trials and basic pharmacodynamic studies, chlorogenic acid for injection demonstrated strong anti-tumor activity against small cell lung cancer and squamous cell lung cancer. Under the leadership of the Cancer Institute and Hospital of Chinese Academy of Medical Sciences, Jiuzhang Biotech had carried out clinical studies on chlorogenic acid for injection for treatment of advanced lung adenocarcinoma, squamous cell lung cancer and small cell lung cancer to further expand the indications and clinical application of chlorogenic acid for injection.
Currently, the development of clinical protocol and screening of clinical center hospitals have been completed, and all the subjects to be enrolled are patients with advanced lung cancers who have metastasis after failure to conventional therapy without available effective drugs, especially small cell lung cancer.







