This study was conducted in accordance with the requirements of the clinical approval document (CFDA2013L01855). It was a non-randomized, open-label, single-dose, and continuous dose-escalation Phase I clinical trial, designed to evaluate the safety, tolerability, and pharmacokinetics of CHA for injection in treating advanced malignant glioma. Additionally, dose expansion studies were conducted in dose groups where subjects were likely to benefit, to preliminarily observe the safety and efficacy of the drug.
Trial objectives:
To observe the tolerability of CHA for injection in humans and to determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of CHA for injection in patients with advanced malignant glioma.
Target group:
Patients with recurrent high-grade (WHO Grade III-IV) glioma who have failed standard treatments.
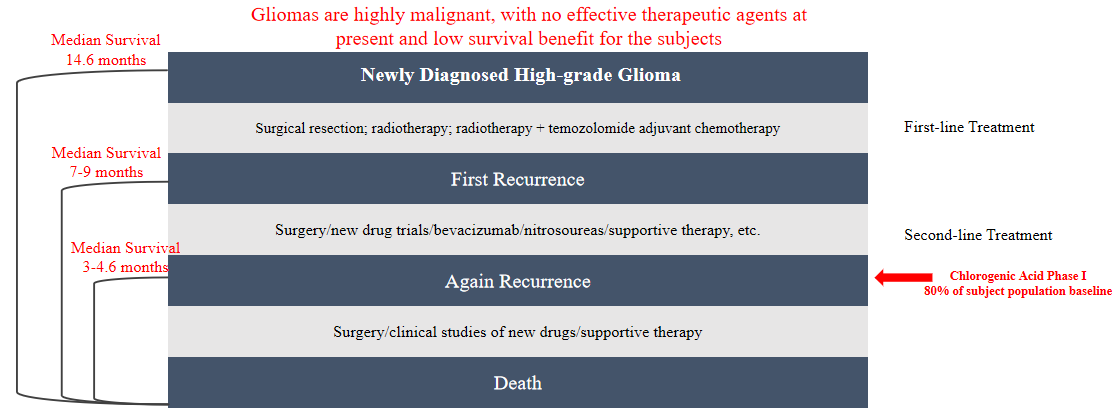
Clinical summary::
1、Safety
Tolerability results showed that CHA for injection demonstrated good tolerability in both single and multiple intramuscular injections.
Safety results showed that in all dose groups of single and multiple intramuscular injections, the primary drug-related adverse reaction was muscle induration at the injection site. Most subjects were able to tolerate continued administration after the occurrence of induration, indicating that CHA for injection was safe.
Pharmacokinetic results showed that after multiple intramuscular injections of CHA for injection, the prototype drug was rapidly metabolized and excreted in the human body, with no tendency for accumulation, suggesting its suitability for long-term use.
2、Efficacy
According to the preliminary efficacy evaluation on CHA for injection, the median overall survival (OS) in the effective dose group was 3.6 to 6 times greater than that documented in the literature for subjects with recurrent glioblastoma multiforme (GBM), significantly prolonging the survival of advanced patients.
Most patients with recurrent high-grade glioma who have failed standard treatments are in an irreversible state of stable disease-progressive-disease (SD-PD) death. Complete response (CR) or partial response (PR) is rare (a low-probability event). However, in this trial, 2 subjects achieved CR and PR, with significant therapeutic responses far exceeding researchers’ expectations.
CHA for injection demonstrated good medication compliance, a clear pharmacokinetic pathway, and no cumulative toxicity. The effective dose for treating advanced recurrent high-grade malignant glioma has been preliminarily determined.


