Glioma
Navigation
Typical cases:
Phase I Special Case Analysis : CHA for Injection relieves TMZ resistance in subjects to some extent
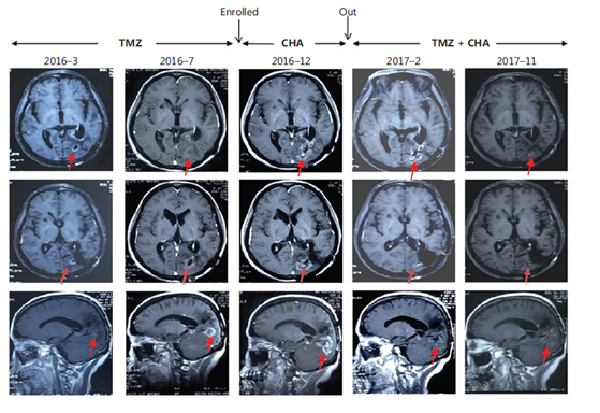
Subject 005 had progressive disease and TMZ resistance when enrolled in the group.
The use of CHA was followved by the re-administration of'T'MZ in Feb. 2017, and MRl results showed that TlZ effected again
The patient has continued the medication to date, and the intracranial lesion was in complete remission during re-examination in
December 2023, with a survival of 12 years from diagnosis.
Source of case image: Kang, Z., Li, S., Kang, X., Deng, J., Yang, H., & Chen, F., et al. (2023). Phase I study of CHA injection for recurrent high-grade glioma with long-term follow-up. Cancer Biology & Medicine, 20(6), 465-476.
Patent link:
Publication link:
-
Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up
 Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up2023.05.20This study was aimed at analyzing the efficacy and safety of an injectable form of CHA in patients with recurrent high-grade glioma after standard of care treatments, through a first-in-human, open-label, dose-escalation phase I trial.
Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up2023.05.20This study was aimed at analyzing the efficacy and safety of an injectable form of CHA in patients with recurrent high-grade glioma after standard of care treatments, through a first-in-human, open-label, dose-escalation phase I trial. -
First-in-human immunomodulation mechanism research of chlorogenic acid in patients with recurrent high-grade glioma
 First-in-human immunomodulation mechanism research of chlorogenic acid in patients with recurrent high-grade glioma2018.04.27This study presented evidence about the immunomodulation function of CHA in patients with recurrent high-grade glioma. In CHA responsive patients, CHA may change the gene expression and upregulate the immune system activity to inhibit tumor development, thereby making it a potential antitumor drug.
First-in-human immunomodulation mechanism research of chlorogenic acid in patients with recurrent high-grade glioma2018.04.27This study presented evidence about the immunomodulation function of CHA in patients with recurrent high-grade glioma. In CHA responsive patients, CHA may change the gene expression and upregulate the immune system activity to inhibit tumor development, thereby making it a potential antitumor drug.


