-
 In-depth R&D of CHA
In-depth R&D of CHAStructural modification of CHA
Development of new dosage forms
Synthetic pathway
Other fields
-
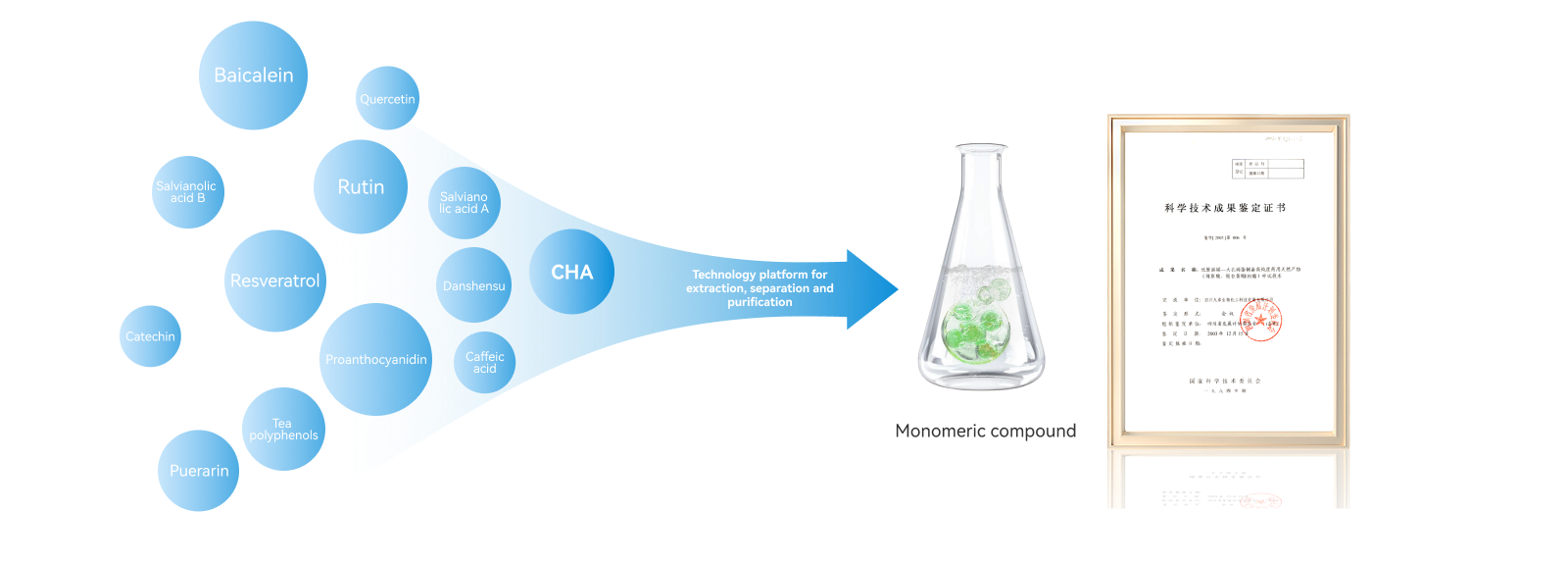
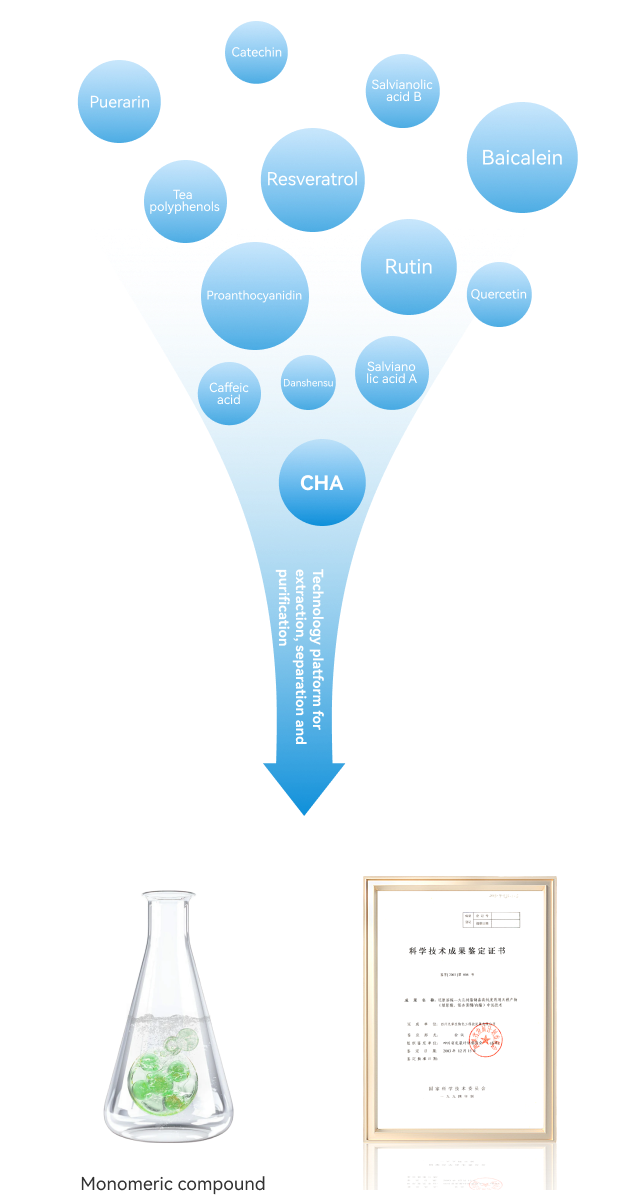
 Natural small molecule drugs
Natural small molecule drugsExploiting medicinal natural small-molecule compounds through the extraction, separation, and purification technology platform for oxidation-prone medicinal natural products
-
 Differentiation-inducing/metabolic-targeting therapeutics
Differentiation-inducing/metabolic-targeting therapeuticsDeveloping other differentiation-inducing/metabolic-targeting therapeutics
-
 Introducing projects
Introducing projectsIntroducing or jointly developing innovative drugs or high-end generic drugs
Jiuzhang Biotech has applied for patent protection for CHA in key therapeutic areas, related derivatives, core processes, new dosage forms, routes of administration, and other aspects.
By Jul. 28, 2025, Jiuzhang Biotech has obtained 53 authorized Chinese patents related to drug patent protection, all of which are invention patents. Additionally, the Company has secured 29 foreign authorized patents, all of which are also invention patents, including 19 in the United States, 5 in Europe, 3 in Japan, 1 in South Korea and 1 in Australia. There are 5 patents in Chinese applications. There are 8 patents pending abroad, including 5 in the United States, 1 in Australia, 1 in Japan, and 1 in South Korea.
Jiuzhang Biotech possesses full independent intellectual property rights without any joint or exclusive authorized use licenses.
Declaration: Among the 211 publicly disclosed patent records of Sichuan Jiuzhang Biotechnology Co., Ltd., any assertions by entities or individuals other than the patent applicants and inventors regarding their involvement in the experimental aspects of the patents, their guidance on drafting, or their planning and organization of the patent applications are hereby declared false.
-
ScRNA-seq unveils the functional characteristics of glioma-associated macrophages and the regulatory effects of chlorogenic acid on the immune microenvironment—a study based on mouse models and clinical practice
 ScRNA-seq unveils the functional characteristics of glioma-associated macrophages and the regulatory effects of chlorogenic acid on the immune microenvironment—a study based on mouse models and clinical practice2025.01.10Glioma is the most common primary malignant brain tumor. Despite advances in surgical techniques and treatment regimens, the therapeutic effects of glioma remain unsatisfactory. Immunotherapy has brought new hope to glioma patients, but its therapeutic outcomes are limited by the immunosuppressive nature of the tumor microenvironment (TME). This study aimed to reveal the subpopulations and functional characteristics of tumor-associated macrophages (TAMs) and explore the regulatory effects of CHA on the immune microenvironment, as well as its potential for clinical application.
ScRNA-seq unveils the functional characteristics of glioma-associated macrophages and the regulatory effects of chlorogenic acid on the immune microenvironment—a study based on mouse models and clinical practice2025.01.10Glioma is the most common primary malignant brain tumor. Despite advances in surgical techniques and treatment regimens, the therapeutic effects of glioma remain unsatisfactory. Immunotherapy has brought new hope to glioma patients, but its therapeutic outcomes are limited by the immunosuppressive nature of the tumor microenvironment (TME). This study aimed to reveal the subpopulations and functional characteristics of tumor-associated macrophages (TAMs) and explore the regulatory effects of CHA on the immune microenvironment, as well as its potential for clinical application. -
Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid
 Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid2024.07.10CHA is a natural product that effectively inhibits tumor growth, demonstrated in many preclinical models, and phase II clinical trials for patients with glioma. However, its direct proteomic targets and anticancer molecular mechanisms remain unknown. Herein, we developed a novel bi-functional photo-affinity probe PAL/CHA and discovered mitochondrial acetyl-CoA acetyltransferase 1 (ACAT1) was one of the main target proteins of CHA by using affinity-based protein profiling (AfBPP) chemical proteomic approach. We performed in-depth studies on ACAT1/CHA interactions via multiple assays including SPR, ITC, and cryo-EM. Importantly, we demonstrated that CHA impaired cancer cell proliferation by inhibiting the phosphorylation of tetrameric ACAT1 on Y407 residue through a novel mode of action in vitro and in vivo. Our study highlights the use of AfBPP platforms in uncovering unique druggable modalities accessed by natural products. And identifying the molecular target of CHA sheds light on the future clinical application of CHA for cancer therapy.
Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid2024.07.10CHA is a natural product that effectively inhibits tumor growth, demonstrated in many preclinical models, and phase II clinical trials for patients with glioma. However, its direct proteomic targets and anticancer molecular mechanisms remain unknown. Herein, we developed a novel bi-functional photo-affinity probe PAL/CHA and discovered mitochondrial acetyl-CoA acetyltransferase 1 (ACAT1) was one of the main target proteins of CHA by using affinity-based protein profiling (AfBPP) chemical proteomic approach. We performed in-depth studies on ACAT1/CHA interactions via multiple assays including SPR, ITC, and cryo-EM. Importantly, we demonstrated that CHA impaired cancer cell proliferation by inhibiting the phosphorylation of tetrameric ACAT1 on Y407 residue through a novel mode of action in vitro and in vivo. Our study highlights the use of AfBPP platforms in uncovering unique druggable modalities accessed by natural products. And identifying the molecular target of CHA sheds light on the future clinical application of CHA for cancer therapy. -
ACAT1 Induces the Differentiation of Glioblastoma Cells by Rewiring Choline Metabolism
 ACAT1 Induces the Differentiation of Glioblastoma Cells by Rewiring Choline Metabolism2024.01.05Abnormal differentiation of cells is a hallmark of malignancy. Induction of cancer-cell differentiation is emerging as a novel therapeutic strategy with low toxicity in hematological malignances, but whether such treatment can be used in solid tumors is not known. Here, we uncovered a novel function of acetyl coenzyme A acetyltransferase (ACAT1) in regulating the differentiation of glioblastoma (GBM) cells. Inhibition of ACAT1 promoted the differentiation of GBM cells into astrocytes but also delayed tumor growth. Mechanistically, suppression of ACAT1 restored mitochondrial function and led to metabolic “reprogramming” in GBM cells: reduction of fatty-acid oxidation and acetyl-CoA, but an increase in free fatty acids. Importantly, ACAT1 negatively regulated the choline metabolic pathway, which is crucial for the differentiation of GBM cells. Finally, we demonstrated that a naturally available substance, CHA, could inhibit phosphorylation of ACAT1 and so delay GBM progression, CHA is a promising candidate to treat GBM because it could induce the differentiation of cancer cells.
ACAT1 Induces the Differentiation of Glioblastoma Cells by Rewiring Choline Metabolism2024.01.05Abnormal differentiation of cells is a hallmark of malignancy. Induction of cancer-cell differentiation is emerging as a novel therapeutic strategy with low toxicity in hematological malignances, but whether such treatment can be used in solid tumors is not known. Here, we uncovered a novel function of acetyl coenzyme A acetyltransferase (ACAT1) in regulating the differentiation of glioblastoma (GBM) cells. Inhibition of ACAT1 promoted the differentiation of GBM cells into astrocytes but also delayed tumor growth. Mechanistically, suppression of ACAT1 restored mitochondrial function and led to metabolic “reprogramming” in GBM cells: reduction of fatty-acid oxidation and acetyl-CoA, but an increase in free fatty acids. Importantly, ACAT1 negatively regulated the choline metabolic pathway, which is crucial for the differentiation of GBM cells. Finally, we demonstrated that a naturally available substance, CHA, could inhibit phosphorylation of ACAT1 and so delay GBM progression, CHA is a promising candidate to treat GBM because it could induce the differentiation of cancer cells.


