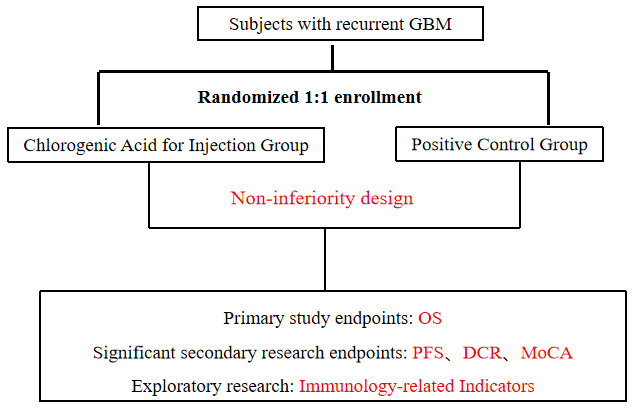
This study was conducted in accordance with the requirements of the clinical approval document (CFDA2013L01855). It was a randomized, controlled, open, multicenter, phase II / III clinical trial to evaluate the safety and efficacy of chlorogenic acid for injection in the treatment of recurrent grade IV glioblastoma ( GBM ) .
Trial objectives:
Evaluating Overall Survival (OS) of patients with Grade IV Glioblastoma (GBM) treated with Chlorogenic Acid for Injection.
Target group:
Patients with recurrent grade IV GBM who were ineffective after standard treatment ( surgery, radiotherapy and temozolomide chemotherapy ) and were diagnosed after screening examination.
Clinical Protocol Design:
Progress:
Central progress
|
Number of clinical centers
|
Center name
|
|
launch |
11 |
Beijing tiantan hospital. capital medical university; Harbin medical university cancer hospital; Chongqing cancer hospital; Union hospital tongji medical college huazhong university of science and technology; General hospital of ningxia medical university; Jiangsu province hospital; Tianjin huanhu hospital; Shenzhen second people's hospital; Xi 'an tangdu hospital; Sichuan provincial people's hospital; Peking union medical college hospital. |


