Objective: This study was aimed at analyzing the efficacy and safety of an injectable form of CHA in patients with recurrent high-grade glioma after standard of care treatments, through a first-in-human, open-label, dose-escalation phase I trial.
Methods: A total of 26 eligible patients were enrolled, received intramuscular CHA injections at 5 dose levels, and were followed up for 5 years. CHA was well tolerated, and the maximum tolerated dose was 5.5 mg/kg.
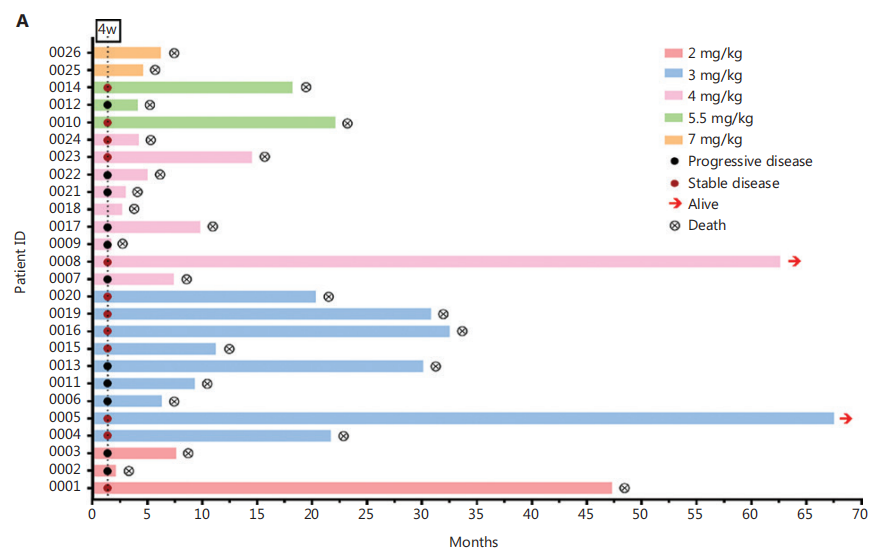
Results: The most common treatment-related adverse events occurred at the sites of injection. No grade 3 or 4 adverse events (e.g., drug allergy) were reported for these patients except for induration at the injection sites. A clinical pharmacokinetic study showed that CHA was rapidly eliminated from the plasma, with a t1/2 of 0.95–1.27 h on day 1 and 1.19–1.39 h on day 30, and no detectable CHA was observed on days 9, 11, 13, 23, 25, 27, and 29 before CHA administration. After the first treatment cycle, 52.2% of patients (12 of 23) achieved stable disease. Long-term follow-up indicated an estimated median overall survival of 11.3 months for all 23 evaluable patients. Of the 18 patients with grade 3 glioma, the median overall survival was 9.5 months. Two patients remained alive at the cutoff day.
Conclusions: This phase I study demonstrated that CHA has a favorable safety profile (with no severe toxicity), and provides preliminary clinical benefits for patients with high grade glioma relapsing after prior standard therapies, thus shedding light on the potential clinical application of CHA for recurrent grade 4 glioma.
Days of CHA treatment at the extended time
Literature link
Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up



