Cell Metabolism: The "food source" of cancer cells is anticipated to be cut off by the Fasting Mimicking Diet (FMD).
Has the term "fasting" not been discussed for some time? The last discussion of fasting and cancer treatment by Qidian Gao (a self-designated name, likely referring to a person or entity related to the content) occurred nearly two years ago. The decline in the popularity of fasting is largely attributable to its limited success in its primary application—weight loss. However, as one door closes, another may open. The exploration of fasting as a potential strategy in cancer treatment represents a promising new direction.
The results of the phase II BREAKFAST clinical study have been officially published by the team from the National Cancer Institute in Milan, Italy, in the journal Cell Metabolism [1]. It has been confirmed that a Fasting-mimicking Diet (FMD) with strict calorie restriction, combined with standard neoadjuvant chemotherapy, can achieve an excellent pathological complete response (pCR) rate in patients with early-stage triple-negative breast cancer (TNBC). This effect can be translated into long-term survival benefits for patients. Additionally, the treatment has been found to be safe and tolerable.
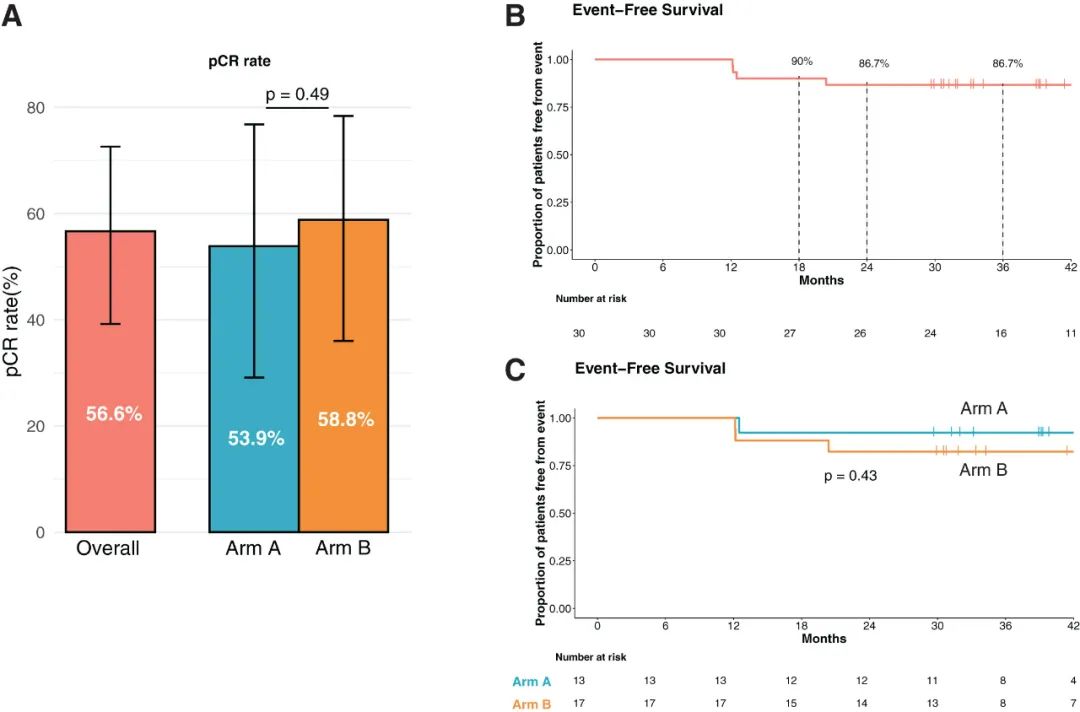
The research results demonstrate that a Fasting-mimicking Diet (FMD) administered for 5 days per week over 3-week cycles, in conjunction with chemotherapy (± metformin), can achieve a pathological complete response (pCR) rate of 56.6%. This efficacy is comparable to that observed in key clinical studies combining neoadjuvant immunotherapy and chemotherapy. The 3-year event-free survival (EFS) rate among patients reaches 86.7%, significantly surpassing historical data. Multi-omics analysis reveals that the FMD downregulates tumor glycolysis and pyruvate metabolism levels, which may underlie the clinical benefits observed.
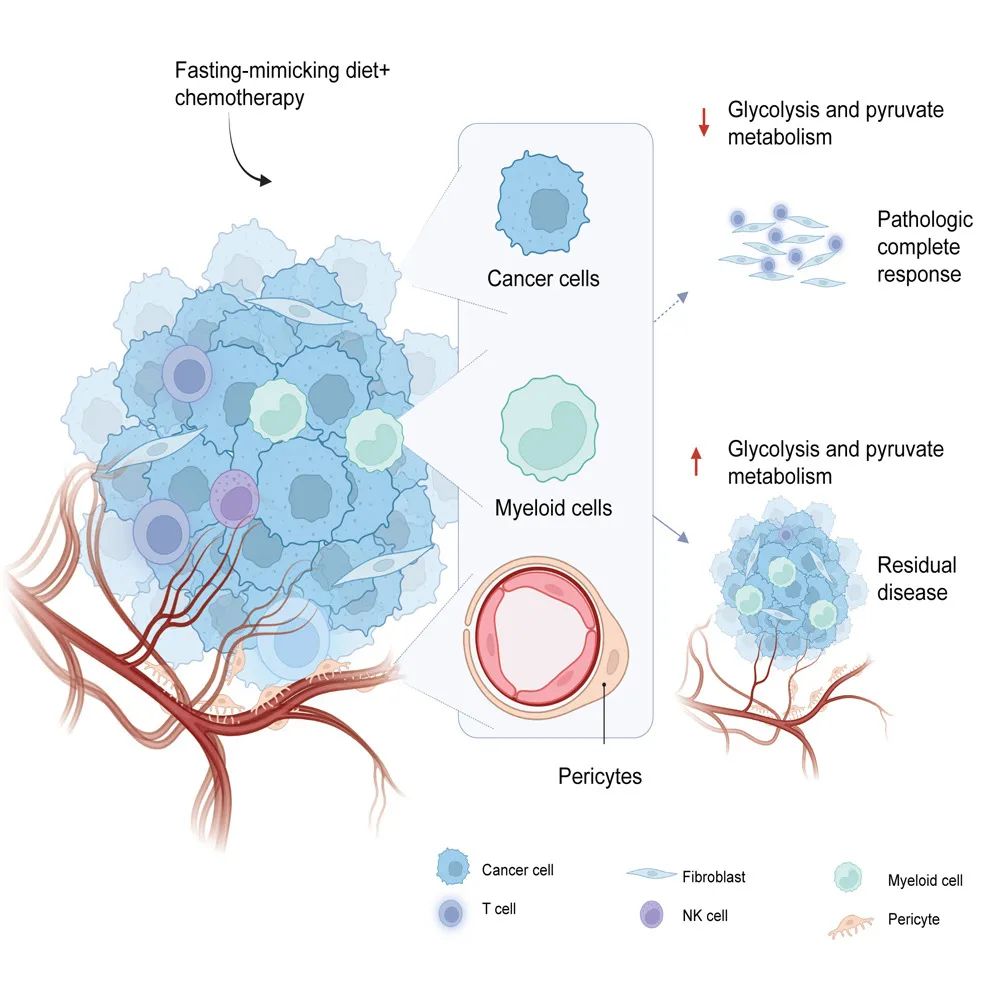
The principle underlying the use of fasting and the Fasting-mimicking Diet (FMD) in cancer treatment primarily involves two mechanisms. Firstly, by reducing nutrient levels in the tumor microenvironment, cancer cells are directly subjected to "starvation," rendering triple-negative breast cancer (TNBC)—characterized by a high "appetite for sugar"—particularly sensitive to FMD intervention. Secondly, the FMD modulates the anti-tumor immune response. For instance, a preclinical study published by the Italian research team in 2022 demonstrated that an FMD with strict calorie restriction, low carbohydrate, and low protein intake can reshape the intratumoral immune microenvironment of breast cancer, including TNBC, thereby enhancing the efficacy of chemotherapy [2].
The Fasting-mimicking Diet (FMD) protocol employed in the BREAKFAST study is as follows: A total of 30 patients were permitted to consume 600 kilocalories on the first day of each FMD cycle, with calorie intake restricted to 300 kilocalories from the second to the fifth day. The diet consisted primarily of plant-based foods with low protein content. Patients were allowed to undergo a maximum of eight 3-week FMD cycles. The FMD was discontinued if patients required chemotherapy delays due to recurrent adverse events or if their body mass index (BMI) did not reach 20 kg/m² prior to the start of an FMD cycle. The latter scenario was the primary reason for discontinuation among the enrolled patients (7 out of 8 cases).
The calorie-restricted FMD protocol, while appearing stringent, has been verified for its feasibility and tolerability in a 2022 study. Although 70% of the patients reported grade 3-4 adverse events (AEs) during the study, most were attributed to chemotherapy. Only one patient experienced grade 3-4 AEs related to the FMD. Additionally, 22 patients completed eight cycles of FMD combined with chemotherapy, and 19 patients adhered to the prescribed diet plan without requiring individual adjustments.
Based on the Fasting-mimicking Diet (FMD), the enrolled triple-negative breast cancer (TNBC) patients in the study were divided into two groups and received neoadjuvant chemotherapy consisting of anthracycline + taxane ± metformin (groups A and B). The overall pathological complete response (pCR) rate among the patients reached 56.6%, which exceeded the pCR rate typically observed with standard neoadjuvant chemotherapy in most phase III clinical studies (approximately 15-50%). This rate was also comparable to that observed in the KEYNOTE-522 study, where immunotherapy combined with chemotherapy was administered to TNBC patients (64.8%). No significant difference in the pCR rate was observed between groups A and B.
The combination of the Fasting-mimicking Diet (FMD) and neoadjuvant chemotherapy has resulted in a significant improvement in long-term prognosis. After a median follow-up period of more than 3 years (39.3 months), only 4 out of 30 enrolled patients experienced recurrence. The 36-month event-free survival (EFS) rate reached 86.7%, and the overall survival (OS) rate exceeded that of previous studies employing simple neoadjuvant chemotherapy. In prior studies, the 3-year EFS rate was approximately 65%, and the OS rate was approximately 80%.
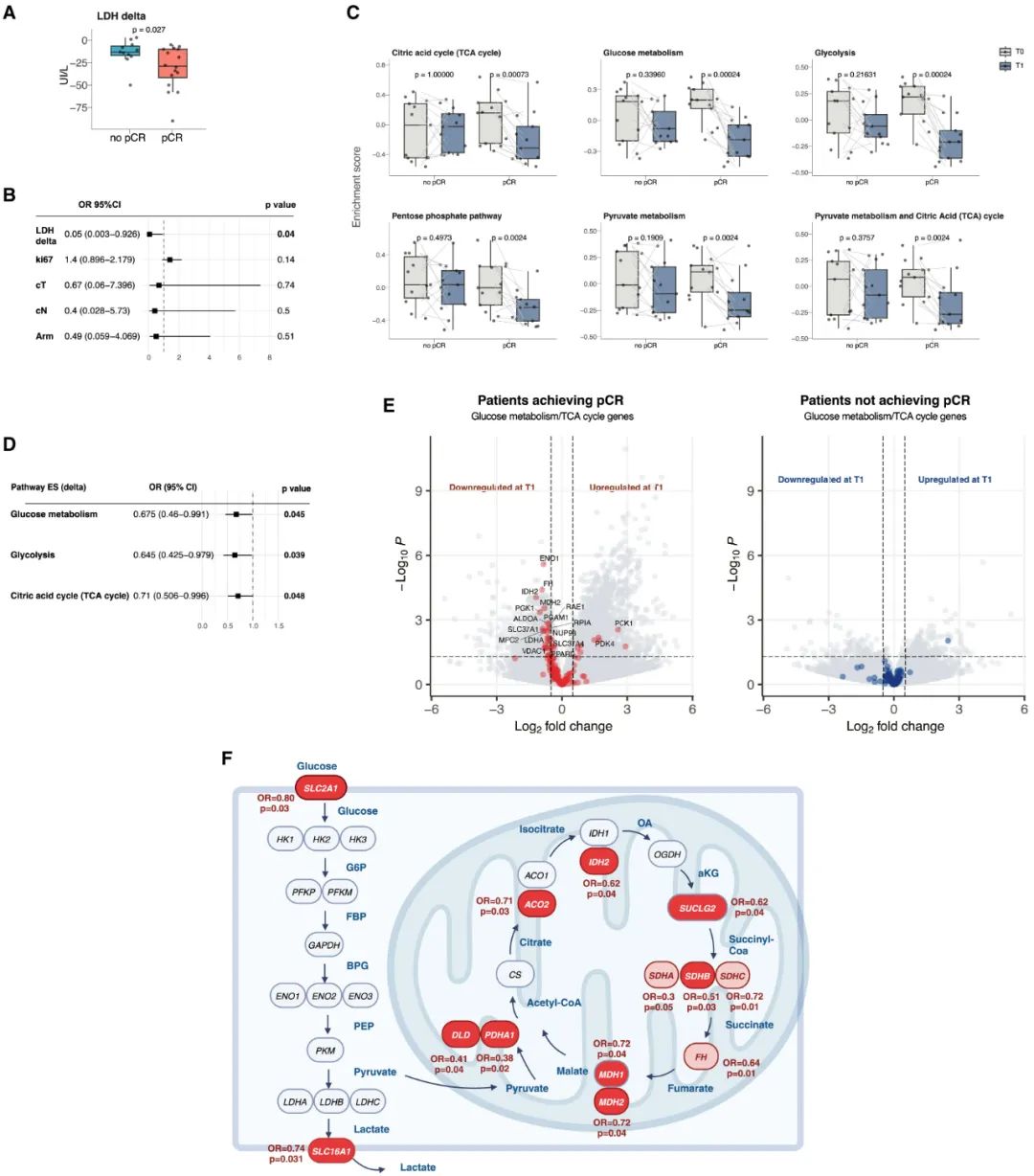
In line with the successful completion of the Fasting-mimicking Diet (FMD) by most patients, significant changes were observed in body mass index (BMI), body fat level, and multiple metabolic indicators both before and after treatment. Notably, a marked decrease in lactate dehydrogenase (LDH) levels was observed, suggesting that the FMD effectively inhibits tumor glycolysis.
Single-cell RNA sequencing and transcriptomic analysis of the surgically resected tumor samples revealed that the glycolytic capacity and pyruvate metabolism levels of cancer cells and certain intratumoral cells with high glycolytic activity (such as pericytes) were significantly downregulated shortly after initiating the Fasting-mimicking Diet (FMD) combined with neoadjuvant chemotherapy. Concurrently, immune infiltration within the tumor microenvironment was significantly enhanced. Additionally, early downregulation of glycolysis emerged as an independent predictor of pathological complete response (pCR) following treatment.
If there is any regret regarding the BREAKFAST study, it is likely that it was "unfortunately initiated at an inopportune time," as the KEYNOTE-522 study achieved positive results during the enrollment period. This led to the adoption of neoadjuvant immunotherapy combined with chemotherapy as the new standard clinical treatment for triple-negative breast cancer (TNBC). However, Italian researchers have initiated the BREAKFAST-2 study to evaluate the combination of the Fasting-mimicking Diet (FMD) with immunotherapy and chemotherapy. Should the triple combination of fasting, chemotherapy, and immunotherapy prove successful, cancer cells may be effectively eradicated through starvation.
Disclaimer: This article is only for sharing. If there are any issues related to copyright, please contact us as soon as possible. We will correct it immediately. Thank you!


