Cancer as a Metabolic Disease: Cell Studies Reveal Arginine-Driven Metabolic Reprogramming in Liver Cancer Growth
The liver, a vital organ in the human body, is responsible for numerous critical functions. Nutrient metabolism, energy storage, and blood sugar regulation are among these functions. Additionally, the liver plays a pivotal role in detoxification and the elimination of harmful substances and drugs.
Liver cancer is recognized as one of the deadliest malignancies globally. The 2020 Global Cancer Burden Data, which was released by the International Agency for Research on Cancer (IARC) under the World Health Organization (WHO), indicates that liver cancer ranks as the sixth most common cancer in terms of incidence and the third leading cause of cancer-related deaths. The highest burden of liver cancer is borne by China, which accounts for nearly half of both new cases and deaths annually. Obesity, alcohol consumption, and hepatitis virus infections are noted to be strongly associated with the development of liver cancer. Early diagnosis and appropriate treatment strategies are deemed crucial for improving patient outcomes.
Among liver cancers, hepatocellular carcinoma (HCC) is the most prevalent type, accounting for 90% of primary liver malignancies. This aggressive cancer is characterized by difficult early detection and rapid progression. Most patients are diagnosed at advanced stages, resulting in limited effective treatment options and a five-year survival rate of only 15-18%.
Cancer cells exhibit "chameleon-like" adaptability, radically altering their metabolism to sustain uncontrolled growth. Elevated levels of the amino acid arginine have recently been discovered to drive metabolic reprogramming, thereby promoting tumor growth. This finding was made by scientists at the University of Basel in Switzerland.
The study, titled "Arginine reprograms metabolism in liver cancer via RBM39," was published on October 6, 2023, in the prestigious international journal Cell.

Cancer is recognized as a metabolic disorder
Over the past decade, significant advances have been made in understanding multiple aspects of cancer. Historically, cancer has been viewed primarily as a disorder of cell proliferation.
However, accumulating evidence suggests that cancer is fundamentally a metabolic disease. In other words, cancer arises when cells reprogram their metabolism to enable uncontrolled proliferation.
But how exactly do cells alter their metabolism, and how do these metabolic changes contribute to tumor formation?
In a Cell study, a key driver of metabolic reprogramming in liver cancer cells was identified by a research team led by Professor Michael Hall at the University of Basel.
Arginine accumulation in hepatocellular carcinoma
During the transformation into cancerous cells, healthy liver cells gradually alter their behavior. Their metabolism is reprogrammed to enable rapid growth, for instance by consuming significantly more glucose than normal cells and enhancing nutrient uptake.
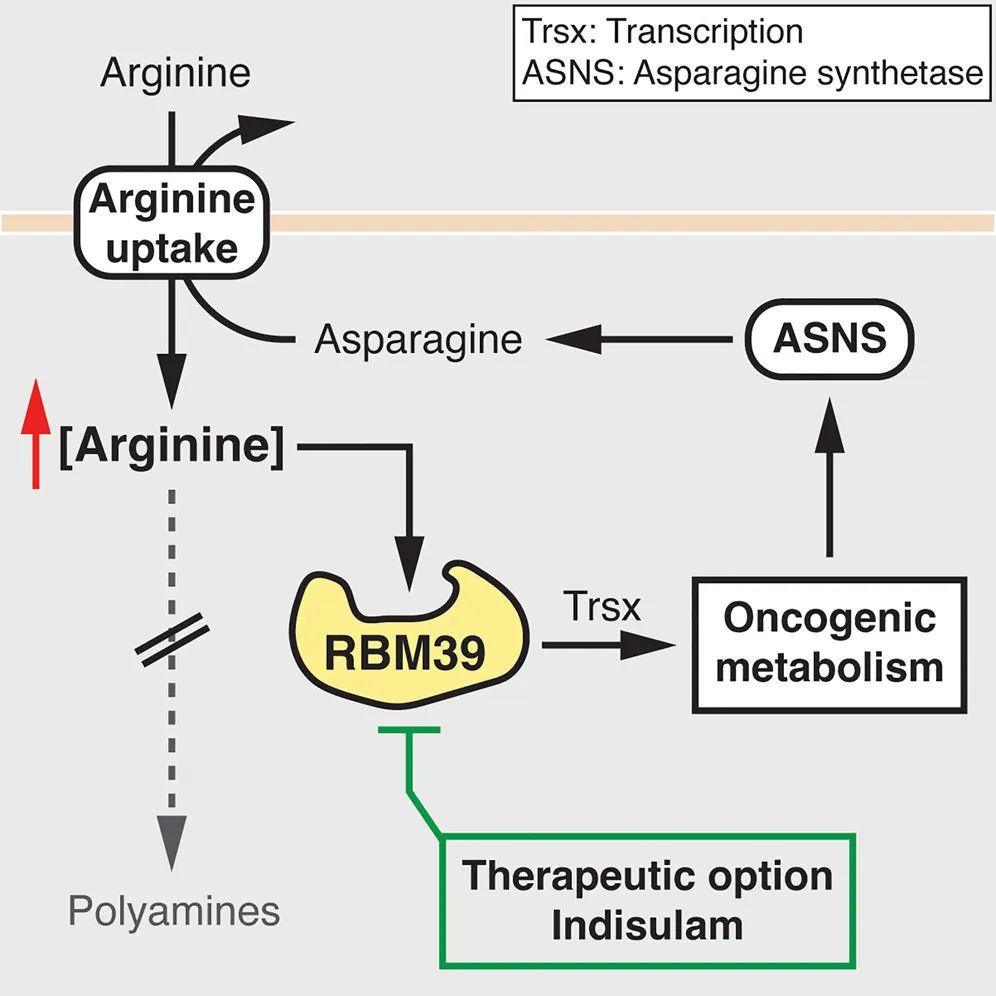
Dr. Dirk Mossmann, the paper's first author, stated: "Hepatocellular carcinoma samples from both mice and human patients were examined, and markedly elevated arginine levels were observed. However, cancer cells produce little to none of this amino acid. High arginine concentrations in tumor cells are accumulated by increasing its uptake while suppressing its catabolism. Furthermore, it was demonstrated that elevated arginine levels are essential for tumor development, independent of its role in protein synthesis."
These findings raise a crucial question: How does arginine contribute to tumor formation?
The role of arginine in tumor growth
High concentrations of arginine were found to combine with specific factors to trigger metabolic reprogramming by regulating the expression of metabolism-related genes, thereby promoting tumor growth. Under such metabolic reprogramming, tumor cells revert to an undifferentiated embryonic cell state, allowing them to divide indefinitely.
Additionally, increased arginine uptake benefits tumor cells in another way. Immune cells rely on arginine for normal functioning, and thus the tumor's uptake of arginine helps tumor cells evade the immune system.
Elevated arginine levels were revealed in both mouse and human hepatocellular carcinoma (HCC) in this study. Tumor cells accumulate high arginine concentrations due to increased arginine uptake and reduced conversion of arginine to polyamines. Importantly, high arginine levels promote tumor formation through further metabolic reprogramming, including alterations in glucose, amino acid, nucleotide, and fatty acid metabolism. Mechanistically, arginine regulates the expression of metabolism-related genes by binding to RNA-binding motif protein 39 (RBM39). The RBM39-mediated upregulation of asparagine synthesis enhances arginine uptake, forming a positive feedback loop that maintains high arginine levels and oncogenic metabolism. Thus, arginine acts as a second messenger-like molecule that reprograms metabolism to promote tumor growth.
Implications of These Findings for HCC Diagnosis and Treatment
Can the role of arginine in driving oncogenic metabolism be leveraged for cancer diagnosis and therapy?
A novel therapeutic strategy was proposed by the research team—targeting cancer-specific arginine-binding factors (such as RBM39) rather than depleting arginine itself. This approach avoids adverse effects on T cells, which require arginine for activation.
Indisulam, a carbonic anhydrase inhibitor known to specifically degrade RBM39, was used by the team to treat hepatocellular carcinoma. The results showed that Indisulam induced RBM39 degradation and prevented metabolic reprogramming. This method circumvents the immunosuppressive side effects caused by systemic arginine depletion.
Moreover, metabolic alterations, such as elevated arginine levels, could serve as biomarkers for early cancer detection, which is critical for successful treatment and patient survival.
In summary, this study—conducted in mice, cell models, and patient-derived HCC organoids—demonstrates that arginine reprograms metabolism in hepatocellular carcinoma by binding to RBM39. This discovery not only provides a new biomarker for early HCC diagnosis but also identifies a promising therapeutic target.


