Clinical Trial Recruitment | The Phase II Clinical Trial of CHA Led by Beijing Tiantan Hospital Has Been Launched
After being reviewed and approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated to Capital Medical University (Ethics Review No. YW2018 - 018 - 01/02/03), the clinical trial titled "A Randomized, Controlled, Open - Label, Multi - center, Phase II/III Clinical Study to Evaluate the Safety and Efficacy of CHA for Injection in the Treatment of Recurrent Grade IV Glioblastoma (GBM)", presided over by Professor Li Wenbin, Director of the Comprehensive Treatment Ward for Neuro - Oncology in the Department of Neurosurgery of Beijing Tiantan Hospital, has been officially launched. After the Spring Festival in 2019, recruitment of recurrent glioblastoma patients who meet the inclusion criteria will officially start from the general public.

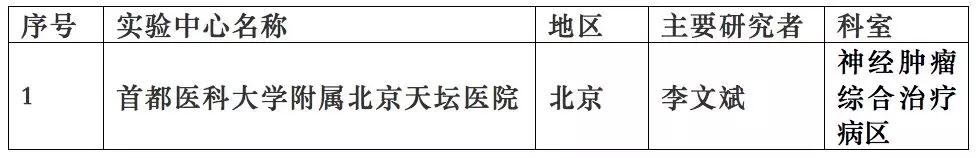
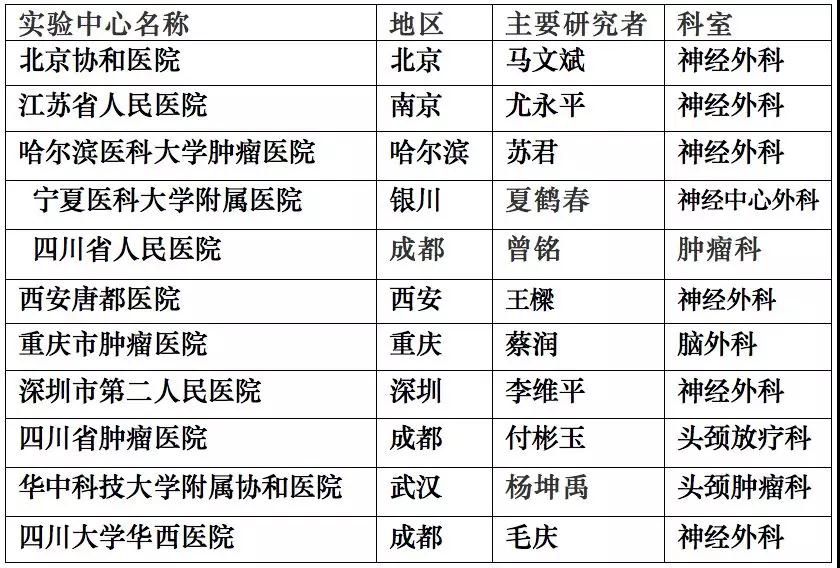
This study has been approved by the China Food and Drug Administration (Approval Document No.: 20153L01855), and the trial will be successively carried out in 12 hospitals across the country with Beijing Tiantan Hospital Affiliated to Capital Medical University taking the lead (see the table below for details).
The trial centers that have been launched:
The trial centers that are about to be launched:
Clinical Trial Information
1.Major inclusion criteria
(1) Aged 18 years or above, regardless of gender;
(2) Histologically confirmed as GBM; patients who have failed to respond to standard treatments including surgery, radiotherapy and temozolomide, and have been diagnosed as having recurrent GBM this time;
(3) Having undergone enhanced cranial MRI and magnetic resonance spectroscopy within 2 weeks, and having at least one measurable tumor lesion.
(4) Karnofsky Performance Status (KPS) score of ≥ 60 points;
(5) Expected survival period of ≥ 3 months;
(6) Female subjects must not be breastfeeding, and must have a negative result in the blood pregnancy test conducted within 7 days before the first administration.
(7) Voluntarily participating in this study and signing the informed consent form, and being able to understand and comply with all the requirements of the study.
2、Trial grouping design
Trial classification: Randomized, controlled, open-label
Number of participants: 200 cases
Eligible subjects who pass the screening can enter this study. According to the dosing regimen, they will be randomly assigned to the experimental group or the control group to receive the corresponding drug treatment. The experimental group uses CHA for injection, administered by intramuscular injection for 28 consecutive days, followed by a 7-day drug-free period. One dosing cycle is 5 weeks. The control group uses lomustine, administered orally at a dose of 110 mg/m², once every 6 weeks, with a maximum of 4 treatment cycles.
3、Risk and Benefit
By participating in this research project, subjects may potentially experience an extension of their overall survival time and an improvement in their quality of life. Moreover, through the research of this project, they can have a clearer understanding of their own disease conditions, which is beneficial for personalized treatment and various nursing support. However, there is no guarantee that they will definitely benefit from the treatment, and patients may also deteriorate due to ineffective treatment or some other potential diseases. The latest information obtained from this research on the treatment of glioma with CHA for injection will be beneficial to the treatment of patients with similar diseases.
4、Relevant Explanations
The examination fees and outpatient/inpatient fees involved for the subjects participating in this clinical study are free of charge. The project team will provide the treatment medications (including CHA for injection and lomustine) free of charge. During the follow-up process, a certain amount of transportation subsidy will be provided. The project team will provide clinical trial insurance for the subjects, which is used to cover the treatment costs and corresponding economic compensation for the subjects who suffer from damages or death related to the trial.


