Release of Phase I Clinical Trial Data on the Safety, Tolerability and Pharmacokinetics of CHA for Injection in the Treatment of Advanced Malignant Gliomas
The "2nd China Pharmaceutical Innovation and Investment Conference" was held at the Suzhou Jinji Lake International Convention Center on October 29, 2017. Jiuzhang Biotech released the Phase I clinical trial data on the safety, tolerability and pharmacokinetics of CHA for injection in the treatment of advanced malignant gliomas.
In 1897, two British scientists, Osbome and Campbell, discovered a compound in sunflowers that could cause the blackening of sunflower seed protein. In 1909, Corter et al. named this compound CHA. In 1947, Rud - kin and Nelson determined the chemical structure of CHA. 103 years later, in 2000, Chinese scientific researchers, the research team of Sichuan Jiuzhang Biotechnology Co., Ltd. (hereinafter referred to as Jiuzhang Biotech) for the first time started the systematic research and development of CHA monomer as a drug for the treatment of major diseases (malignant tumors). In 2013, it obtained the clinical approval document (Class 1 chemical drug) issued by the CFDA.
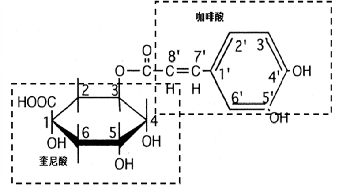
CHA is a kind of phenylpropanoid compound produced during the aerobic respiration process of plants (via the shikimic acid pathway). It is abundant in plants of Caprifoliaceae and Eucommiaceae families.
Based on the kilogram - scale production of high - purity CHA, Jiuzhang Biotech identified and isolated the sensitizing plant protein, qualitatively and quantitatively analyzed the 0.1% impurities in CHA, and successfully transformed the CHA extract into CHA monomer raw material drug. Academically and medically, it has been clarified that CHA is not an allergen and is a natural drug with extremely high development value.
"CHA for injection" belongs to a natural small - molecule immunotherapeutic agent. It has strong pharmacological activity, low toxic and side effects, clear targets, and a clear mechanism of action.
As of December 31, 2017, Jiuzhang Biotech has obtained a total of 24 authorized invention patents and 1 utility model patent in the fields of production technology, structural modification, and disease treatment.
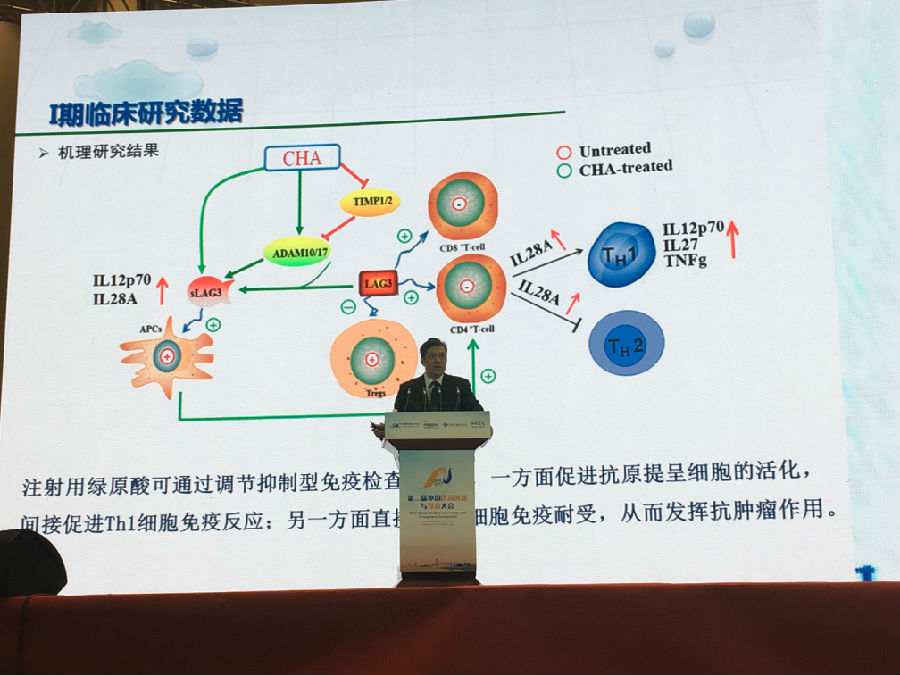
In terms of the research on the efficacy and molecular mechanism of CHA, Jiuzhang Biotech has established a strategic partnership with the Institute of Materia Medica of the Chinese Academy of Medical Sciences, jointly building a high - end platform for the R & D of CHA. Professor Chen Xiaoguang (a well - known domestic expert in pharmacodynamics and pharmacology, a doctoral supervisor) and his team have conducted in - depth research on the anti - tumor mechanism at multiple levels. In January 2017, an article titled "CHA Inhibits the Growth of Malignant Glioma Cells by Repolarizing M2 Macrophages into M1 Macrophages" was published in "Scientific Reports", which elaborated and demonstrated in detail the mechanism by which CHA for injection promotes the apoptosis of malignant tumor cells and inhibits tumor growth. The latest research results on the mechanism of action show that CHA, by regulating the immune checkpoint LAG - 3, on the one hand promotes the activity of antigen - presenting cells, and on the other hand reverses T - cell immune tolerance, thus improving the tumor immune microenvironment and exerting anti - tumor effects. It has extremely low toxic and side effects and is broad - spectrum.
At Beijing Shijitan Hospital, the Phase I clinical trial of "Safety, Tolerability and Pharmacokinetic Study of CHA for Injection in the Treatment of Malignant Gliomas" led by Professor Li Wenbin (Principal Investigator, PI) has been completed. All the subjects were patients with high - grade malignant gliomas who had failed international standard treatments such as surgery, radiotherapy and chemotherapy (disease progression). CHA for injection showed good safety and tolerability. The benefit rate of the subjects at the end - stage of this highly malignant tumor course was over 50%. The target lesions in the brain tissue of some subjects shrank or disappeared, and the efficacy was higher than the design expectation of the clinical protocol. Currently, the Phase II clinical research has been launched, striving to serve a large number of cancer patients as soon as possible.
Based on the results of the research on the anti - tumor mechanism, in order to better develop the broad - spectrum anti - tumor value of CHA for injection, and according to the broad - spectrum characteristics of CHA in anti - tumor, under the leadership of Chairman Zhang Jie, the inventor, the team of Jiuzhang Biotech has formulated a Phase Ib/IIa clinical trial protocol for advanced patients with small - cell lung cancer, postoperative recurrence of squamous cell lung cancer and adenocarcinoma of the lung who have failed conventional treatments. Currently, it is ready to enter the implementation stage.


