[Patient Recruitment] CHA for Injection in the Treatment of Advanced Malignant Gliomas
[Patient Recruitment] Phase I Clinical Trial on the Safety, Tolerability and Pharmacokinetics of CHA for Injection in the Treatment of Advanced Malignant Gliomas

1. Introduction to the Investigational Drug
CHA is a non-endogenous polyphenolic small - molecule substance. Pre - clinical studies have shown that chlorogenic acid activates the body's own immune system, enabling the body to produce an immune response and achieve the effect of inhibiting tumor development. The indication of this trial is advanced malignant gliomas.
2.Trial Objectives
Primary Objective: To observe the tolerance of CHA for injection in humans and determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of CHA for injection in patients with advanced malignant gliomas.
Secondary Objectives: To determine the pharmacokinetic characteristics of single-dose/continuous administration of CHA for injection at different dose levels in patients with advanced malignant gliomas. To preliminarily evaluate the effectiveness and effective dose of CHA for injection in the treatment of advanced malignant gliomas.
3.Trial Design
Trial Classification: Safety and Efficacy
Trial Phase: Other
Design Type: Single - arm Trial
Randomization: Randomized
Blinding: Open - label
Number of Trial Participants: 30 - 90
4.Inclusion Criteria
1. Aged 18 years old or above and 65 years old or below, regardless of gender;
2. Karnofsky Performance Status (KPS) score of 40 or above;
3. Patients with advanced malignant gliomas (primary in the brain and spinal cord) who have failed conventional treatment and have been pathologically/cytologically diagnosed, with a World Health Organization (WHO) grade of III to IV;
4. According to the recommendations of the Response Assessment in Neuro-Oncology (RANO) criteria (2010), the dosage of hormones is stable or increased, and there is an increase of more than 25% in enhanced lesions; or it can be determined that the changes in non-enhanced lesions are caused by tumor progression. Even if these lesions are not measurable, they can still be included in the study;
5. Expected survival period of 3 months or more;
6. Hematological indicators are basically normal: white blood cell count of 3.0×10⁹/L or above, absolute neutrophil count of 1.5×10⁹/L or above, platelet count of 100×10⁹/L or above, and hemoglobin of 90g/L or above;
7. Liver function is basically normal: total serum bilirubin of 1.5 times the upper limit of normal (ULN) or below, and transaminases (alanine aminotransferase (ALT)/aspartate aminotransferase (AST)) of 2.5 times the ULN or below;
8. Renal function is basically normal: serum creatinine of 1.5 times the ULN or below;
9. Cardiac function is basically normal, with a left ventricular ejection fraction of 50% or above;
10. Negative urine pregnancy test for women of childbearing age. Subjects of childbearing age (including male subjects) have no pregnancy plan within the next 12 months and voluntarily take effective contraceptive measures, including but not limited to: hormonal contraception, physical contraception, or abstinence;
11. Willingly participate in this study and sign the informed consent form.
5.Exclusion Criteria
1. Not pathologically/cytologically diagnosed;
2. Those who have undergone extensive radiotherapy (> 30% of bone marrow volume);
3. Patients with primary immunodeficiency;
4. Those with toxicity reactions of grade 2 or above (including grade 2) according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 remaining after previous treatment;
5. Those who have used adrenal corticosteroids within 1 week before the first administration of the study treatment and require long-term treatment with adrenal corticosteroids, except for corticosteroid nasal sprays, inhaled corticosteroids and/or topical corticosteroids;
6. Those who need to use other immunosuppressants for a long time, such as immunosuppressive drugs used after organ transplantation;
7. Those who have received chemotherapy within 4 weeks before the first administration of the study treatment, those who have used nitrosoureas or mitomycin within 6 weeks, those who have used tyrosine kinase inhibitors within 2 weeks, and those who have received radiotherapy within 4 weeks before the first administration of the study treatment;
8. Those who have undergone major surgery within 4 weeks before the first administration of the study treatment or those who have undergone a biopsy surgery within 2 weeks before the first administration of the study treatment;
9. Pregnant or lactating women or patients (including men) with a recent fertility plan;
10. Those with a history of drug abuse;
11. Those who are allergic to any component of the study product;
12. Patients who have participated in other clinical studies within 3 months before enrollment;
13. Those with active hepatitis B, hepatitis C, positive HIV test result, and positive syphilis antibody test result;
14. Uncontrolled diabetes;
15. Those who have suffered from other serious comorbidities within 6 months before enrollment, such as myocardial infarction, severe hypertension, or thrombosis, etc.; other uncontrolled comorbidities include, but are not limited to, ongoing or active infections, mental disorders, or other social factors that limit compliance with the protocol;
16. Those with severe neurological diseases or accompanied by severe neurocognitive impairment;
17. Other situations where the investigator deems it inappropriate for the subject to participate in this study.
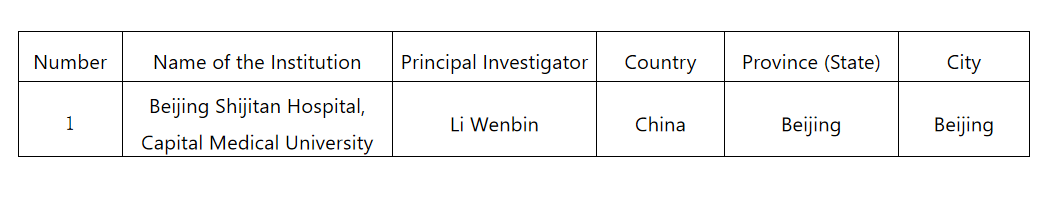
6. Investigator Information